Bioninja Notes - Metabolism
Metabolism
METABOLISM
Metabolism describes the totality of chemical processes that occur within a cell in order to maintain life. These reactions provide a source of energy and enable the synthesis and assimilation of cellular materials. Metabolic reactions are catalysed by enzymes and can be described as being either anabolic or catabolic:
Anabolism:
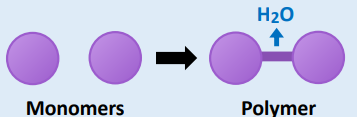
• Smaller compounds are combined to form larger compounds
• In the case of organic compounds, this involves condensation
• Water is released as a by-product of condensation reactions
Catabolism:
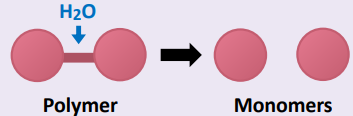
• Large compounds are broken down into smaller compounds
• In the case of organic compounds, this involves hydrolysis
• Water is required as an input for hydrolysis reactions
METABOLIC REACTIONS
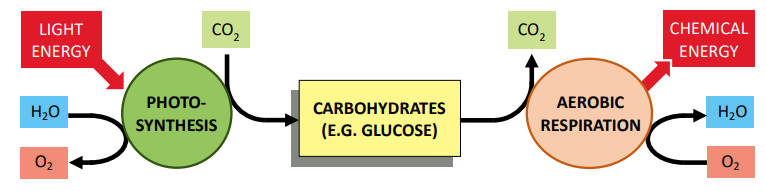
Photosynthesis is an example of an anabolic reaction. It is an endergonic process that uses light energy to synthesise organic compounds from inorganic sources. Conversely, cell respiration is a catabolic reaction. It is an exergonic process that releases chemical energy (ATP) from the breakdown of organic compounds.
ENZYMES
Enzymes control the metabolism of a cell. An enzyme is a globular protein that acts as a biological catalyst. It speeds up the rate of a chemical reaction by lowering the acRvaRon energy threshold required for the reaction to proceed. Enzymes are not changed or consumed by the reactions they catalyse and thus can be reused. Enzymes are commonly named after the molecules they react with (called the substrate) and end with the suffix ‘-ase’ (e.g. lipase is an enzyme that breaks down lipids, whereas proteases digest proteins).
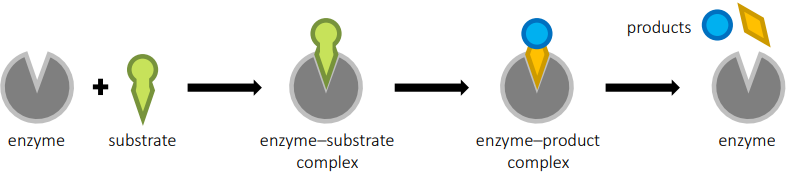
SPECIFICITY All enzymes possess an indentation or cavity to which a substrate can bind – this is called the active site. The shape and chemical properties of the active site are complementary to a particular substrate. Thus, an enzyme demonstrates specificity for a given substrate. Some enzymes are highly specific and have an active site that precisely fits one distinct substrate (the ‘lock and key’ model), while other enzymes may be broadly specific and recognize a class of related molecules (e.g. proteases can digest a variety of proteins). In these instances, the active site undergoes a conformational change to improve bonding (the ‘induce fit’ model). This stresses the bonds in the substrate and increases reactivity (lowers activation energy hurdle).
MOLECULAR MOTION Enzyme reactions occur in aqueous solu:ons (such as the cytoplasm or intersitial fluid), with the substrate and enzyme moving randomly in Brownian motion. For enzyme reactions to occur, a substrate and enzyme must physically collide in the correct orientation to facilitate binding to the active site. The frequency of the successful collisions can be improved by increasing the molecular moRon of the par:cles or increasing the concentration of particles (either substrate or enzyme). Thermal energy can be introduced to increase the kine:c energy of the par:cles, while the enzyme or substrate may occasionally be fixed in a static position (e.g. membrane-bound) to localize reactions to particular sites and increase the likelihood of catalysis.
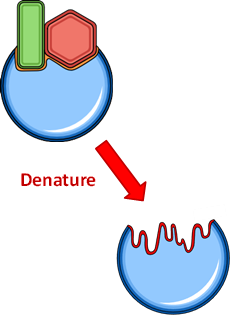
DENATURATION
The shape and chemical properties of the active site are highly dependent on the tertiary structure of the enzyme. This structure can be modified by external factors such as temperature and pH. These factors may disrupt the chemical bonds needed to maintain the tertiary structure, potentially leading to a change in the shape of the active site. This will result in denaturation (loss of biological activity) as the enzyme will no longer be able to interact with the substrate. In most cases, denaturation results in an irreversible loss of biological activity. However, some enzymes may be able to return to a functional state if restored to their native conditions.
In these instances, denaturation is considered to be reversible.
FACTORS AFFECTING ENZYME ACTIVITY
The efficiency of an enzyme-catalysed reaction will be influenced by two key factors:
• The frequency of successful enzyme-substrate collisions (due to more particles or more kinetic motion)
• The capacity for the enzyme and substrate to interact upon collision (will be impacted by denaturation) Factors that affect enzyme activity include: temperature, pH, substrate concentration or enzyme levels
TEMPERATURE
Low temperatures result in insufficient thermal energy for the activation energy threshold to be reached. As temperature increases, particles gain kinetic energy, resulting in more frequent enzyme-substrate collisions. At an optimal temperature, enzyme activity will peak, because higher temperatures will disrupt the bonding within the enzyme, causing a loss of tertiary structure and a resulting loss of biological activity (denaturation).
pH
All enzymes have an optimal pH, at which the activity of the enzyme is at its highest. Outside of this optimal range, enzyme activity will diminish. Amino acids are zwitterions (have both a positive and negative charge), and changing the pH alters the charge of the enzyme (which in turn alters both solubility and overall shape). Enzymes will denature outside of an optimal pH range, leading to a characteristic bell-shaped activity curve.
SUBSTRATE CONCENTRATION
Increasing substrate concentration will increase the activity of a corresponding enzyme. Higher substrate levels will result in increased frequency of collisions with the enzyme in a given period of time. Above a certain substrate concentration, the enzyme activity will plateau. This is because the environment is now saturated with substrate and all enzyme active sites are occupied (reaction is now at maximum catalysis).
ENZYME CONCENTRATION
Increasing enzyme concentration will result in a linear increase in activity. This is because the rate of reaction will be proportional to the amount of enzyme available for reaction (more enzymes will result in more frequent enzyme-substrate collisions, leading to a higher rate of activity). Enzymes will typically exist in low concentrations within living organisms. This is because enzymes are not consumed by the reactions that they catalyse and can continually be reused. Enzymes are generally only used in higher amounts in industrialised settings (e.g. in certain household products or in the generation of chemicals or biofuels).
Cell Respiration
ENERGY
Living organisms need chemical energy to power the metabolic reactions that sustain life processes. These processes include the biosynthesis of organic molecules (via anabolism), the active transport of materials across a cell membrane and the overall movement of cells or cellular components (such as chromosomes).
ADENOSINE TRIPHOSPHATE
A coenzyme is a complex organic molecule that is required for an enzyme’s metabolic activity. Coenzymes cycle between two states: a loaded form capable of assisting enzyme activity and an unloaded form (similar to a charged and expended battery). Adenosine triphosphate (ATP) is a coenzyme that is used to transport chemical energy to metabolic reactions. It functions as an immediate energy source for a cell (i.e. energy currency). ATP possesses three negatively charged phosphates that need increasing amounts of energy to hold together. When the ATP is hydrolysed to adenosine diphosphate (ADP) and an inorganic phosphate, the released energy can then be utilised by the cell
CELL RESPIRATION
Cellular respiration is the controlled release of energy from the breakdown of organic compounds. These compounds are produced by autotrophs (via photosynthesis) or can be synthesised from other pre-existing molecules within the cell (e.g. excess glucose can be converted to fats). Usable carbon compounds include:
• Carbohydrates: The main organic molecule used in cell respiration is the monomer glucose (C6H12O6)
• Triglycerides: Fats produce more energy per gram than sugars, but are harder to transport and digest
• Proteins: Not a primary source as produces nitrogenous by-products (which are toxic if not excreted)
When the organic compounds are broken down in cell respiration, the chemical energy that is released is used to generate ATP (from ADP + Pi). In this respect, the organic compounds function like a bank (storing high amounts of chemical energy within their covalent bonds), while the ATP is usable money. The function of cell respiration is to convert the inaccessible chemical energy into an available form that cells can use.
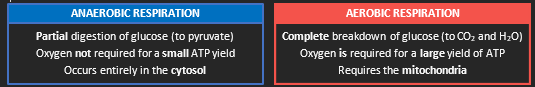
TYPES OF CELL RESPIRATION
Cellular respiration can involve one of two reaction pathways: anaerobic respiration or aerobic respiration
Anaerobic Respiration
Partial breakdown of glucose
Oxygen is not required for a small ATP yield
Occurs entirely in the cytosol
Involves glycolysis and fermentation
Products: Lactic acid / Ethanol + CO2
AEROBIC RESPIRATION
Complete breakdown of glucose
Oxygen is required for a large ATP yield
Occurs in the mitochondria
Involves glycolysis, Krebs cycle and ETC
Products: Carbon dioxide and water
ANAEROBIC RESPIRATION
Anaerobic respiration involves the partial breakdown of carbohydrates (glucose) in the absence of oxygen. It occurs in the cytosol and results in a low yield of ATP (net production = 2 ATP). The glucose can either be converted into lactic acid (animals) or ethanol and carbon dioxide (plants and yeast). The production of lactic acid in humans is used to maximise the power of muscle contractions when levels of oxygen are low.
AEROBIC RESPIRATION
Aerobic respiration involves the complete breakdown of carbohydrates (glucose) in the presence of oxygen. It occurs in the mitochondria and results in a high yield of ATP (net production = 30 ATP). The oxygen is used to complete the digestion of glucose, resulting in the formation of carbon dioxide and water. While sugars like glucose can be digested via either respiratory pathway, triglycerides can only be digested aerobically.
MITOCHONDRIA
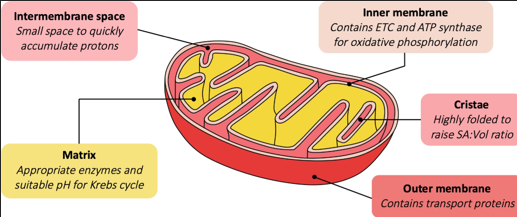
The mitochondrion is an organelle in eukaryotic cells that is responsible for aerobic respiration. It is believed to have evolved via endosymbiosis, when an aerobic bacterium was engulfed by another prokaryotic cell. Evidence for this endosymbiotic origin includes the fact that the mitochondrion possesses circular DNA, 70S ribosomes has a double membrane. In terms of structure, the central region is called the matrix, while the inner membrane is highly folded into cristae. By folding this membrane, the SA:Vol ratio is increased.
This optimises the structure of the mitochondria to allow aerobic respiration to occur more rapidly and in greater amounts (maximising total ATP yield).
FACTORS AFFECTING RESPIRATION RATE
The rate of respiration can be measured by either the consumption of inputs (glucose and oxygen) or the formation of product (carbon dioxide). However, these conditions may be affected by the pathway used. Factors that affect aerobic respiration include temperature and pH (which will alter enzyme functionality), as well as glucose concentration and oxygen availability (both of which function as respiratory substrates).
Photosynthesis
PHOTOSYNTHESIS
Photosynthesis is the process by which cells synthesise organic compounds from inorganic molecules in the presence of sunlight. This process requires a photosynthetic pigment (chlorophyll) and can only occur in certain organisms (plants, algae, cyanobacteria). In plants, photosynthesis occurs within the chloroplast
PIGMENTS
In photosynthetic organisms, the absorption of light is mediated by specific pigment molecules. Each of these pigment molecules contains electrons at discrete and specific energy levels. These electrons absorb light at specific wavelengths and become energised. The energy from these excited electrons is harnessed by the cell to make chemical energy (ATP). In plants, the main photosynthetic pigment is chlorophyll, but other pigments are also used. These pigments are clustered into photosystems to optimise light absorption.
CHROMATOGRAPHY
Chromatography is an experimental technique that allows for the separation of pigments. The pigments are dissolved in a fluid (mobile phase) and passed through a static material (stationary phase). The different pigments will travel at different speeds, causing them to separate. A retardation factor is calculated and the value is used to identify the pigment.
• Rf value = distance the pigment travels (mm) ÷ distance the solvent travels (mm)
The Rf value represents a ratio and hence can never exceed a value of 1. Chromatography experiments will often use paper as the static material and acetone as an organic solvent.
LIGHT ABSORPTION
The electromagnetic spectrum comprises the full range of all types of radiation energy. All photosynthetic organisms will absorb wavelengths within the visible region of this spectrum (white light: 400 – 700 nm). Chlorophyll absorbs red and blue light most effectively, while green light is reflected. This is because green light is too energetic to be used efficiently by the plant, while blue light is best able to penetrate the depths of water (reflecting the likely aquatic origins of living organisms). The amount of light absorbed by pigments at any given wavelength is represented by the absorption spectra, while the rate of photosynthesis at each wavelength is shown by the action spectra. For both spectra, the overall trend is generally comparable.
STAGES OF PHOTOSYNTHESIS
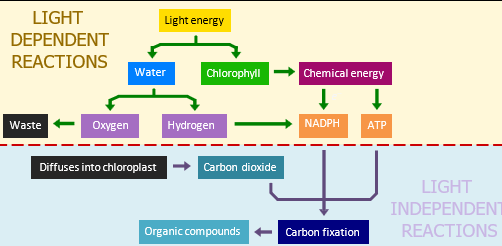
The process of photosynthesis involves two stages: the light dependent reactions and the light independent reactions (Calvin cycle). The light dependent reactions function to convert light energy into chemical energy (ATP) and the light independent reactions then use this chemical energy to synthesise organic compounds. In plants, these reactions occur within a specialised organelle called the chloroplast (found in leaf tissues).
1. LIGHT DEPENDENT REACTIONS
The light dependent reactions require light and occur within the thylakoids (or grana) of the chloroplast. Light is absorbed by photosynthetic pigments (such as chlorophyll), resulting in the excitation of electrons. The energised electrons then transfer this energy to ATP. Simultaneously, light energy breaks down water molecules (photolysis) into oxygen and hydrogen. The oxygen gas is released as a waste product, while the hydrogen atoms will be used (along with ATP) as an input for the light independent reactions (Calvin cycle). The oxygen gas is released from tiny pores on the underside of the leaf called stomata (singular = stoma).
2. LIGHT INDEPENDENT REACTIONS
The second stage of photosynthesis involve the light independent reactions (also called the Calvin cycle). This stage uses the chemical energy (ATP) that was produced in the light dependent stage to synthesise organic compounds (carbohydrates). Molecules of carbon dioxide are combined with hydrogen (from the splitting of water) to form organic molecules like glucose. This anabolic process is catalysed by the enzyme Rubisco and uses the chemical energy from ATP to form the covalent bonds required for carbon fixation. The inputs of the Calvin cycle are carbon dioxide, ATP and hydrogen; while the product formed is glucose. The light independent reactions are localised to the fluid-filled region of the chloroplast (called the stroma).
MEASURING PHOTOSYNTHESIS
Metabolic processes can be measured by the rate of input consumption or the rate of output production. In the case of photosynthesis, the process can therefore be measured by a variety of factors, including:
• Oxygen production: Oxygen gas is a by-product of photosynthesis and can be measured either via the rate of bubble formation (for plants in solution) or via a pressure change in an enclosed container
• Carbon dioxide uptake: Carbon dioxide interacts with water to form an acidic solution (carbonic acid) that lowers pH. Hence, the amount of CO2 production can be measured in solution via a pH indicator.
• Biomass: Organic compounds contribute to the total dry weight of an organism. Hence, the production of glucose can be indirectly measured according to the change in plant mass (grams per dry weight).
LIMITING FACTORS
Photosynthesis is a complex metabolic process that involves several distinct reactions with specific inputs. The law of limiting factors states that when a chemical process depends on multiple essential conditions being favourable, the rate of reaction will be limited by the factor that is nearest to its minimum value.
Photosynthetic rate is influenced by numerous conditions; including temperature, light and carbon dioxide.
CARBON ENRICHMENT EXPERIMENTS
Human activities (such as deforestation and combustion of fossil fuels) are increasing carbon dioxide levels (~400 ppm). Higher levels of carbon dioxide typically increase plant growth and maturation, however excessive concentrations can cause plant damage. Experiments involving enclosed greenhouses and free air carbon dioxide enrichment (FACE) are being undertaken as a way of predicting rates of photosynthesis and plant growth in the future. Enclosed greenhouses allow for greater control of extraneous variables (such as sunlight and water exposure), while FACE experiments can incorporate natural conditions like heat changes.
Enzymes
METABOLIC PATHWAYS
Most chemical changes in a cell result from a series of reactions (metabolic pathways), with each individual step controlled by a specific enzyme. Metabolic pathways can be found within the cytoplasm (intracellular) or outside of the cell (extracellular). Examples of intracellular reactions include the stages of cell respiration (glycolysis and the Krebs cycle), while an example of an extracellular reaction is the breakdown of nutrients within the gut (chemical digestion). Metabolic pathways are typically organised into either chains or cycles of enzyme-catalysed reactions. Linear chains occur in processes such as glycolysis and blood clotting, while cyclical pathways are present in processes such as the Krebs cycle and the Calvin cycle. Metabolic pathways allow for a greater level of regulatory control, as a chemical change is controlled by multiple intermediates.
Chain Cycle
ENERGETICS
Metabolic reactions are not 100% efficient in their energy transfers – a proportion of energy is always lost as heat. Certain animals (such as mammals and birds) use this heat generation to maintain a constant body temperature (endotherms). The level of heat production by these animals can be regulated by controlling their level of metabolic activity. Some animals may even enter a state of torpor to reduce heat production.
INHIBITORS
An enzyme inhibitor is a molecule that binds to an enzyme and disrupts substrate interaction. Inhibitors can be produced by cells to regulate metabolic processes by preventing catalysis. Inhibitors are also introduced from the external environment to damage cells (e.g. venoms). Inhibitors can be categorised based on their mechanism of action (competitive vs non-competitive) or their consequence on cellular activity (feedback inhibition or mechanism-based inhibition). Inhibition can be reversible (by forming weak hydrogen bonds) or irreversible (by forming strong covalent bonds). Inhibitors can be used to treat some disease conditions.
COMPETITIVE INHIBITION
Competitive inhibition involves a molecule, other than the substrate, binding reversibly to an enzyme’s active site. The molecule (inhibitor) is structurally and chemically similar to the substrate. The competitive inhibitor functions to block the active site and thus prevents substrate binding. As the inhibitor is in competition with the substrate, its effects can be reduced by increasing the concentration of the substrate.
Statins are common cholesterol lowering drugs that function as competitive inhibitors. Statins bind to the active site of the enzyme HMG-CoA reductase which forms a part of the metabolic chain responsible for cholesterol synthesis. By blocking this metabolic chain, cholesterol production is reduced, minimising the health consequences associated with high cholesterol levels (such as symptoms of coronary heart disease).
NON-COMPETITIVE INHIBITION
Non-competitive inhibition involves a molecule binding to a site other than the active site (allosteric site). The binding of an inhibitor to the allosteric site causes a conformational
change to the enzyme’s active site. As a result of the change, the active site and substrate will no longer share specificity, meaning the substrate cannot bind. Increasing the substrate levels cannot mitigate inhibitor’s effect (not in competition).
FEEDBACK INHIBITION
Feedback inhibition is a form of negative feedback by which metabolic pathways can be regulated. In feedback inhibition, the final product in a series of reactions inhibits an enzyme from an earlier step in the sequence. The product will bind to an allosteric site and temporarily inactivates the enzyme. As an enzyme can no longer function, the reaction sequence is halted and the product formation rate will be decreased.
An example of feedback inhibition is the regulatory control of isoleucine production in bacteria. Isoleucine may be synthesised from threonine in a multi-step pathway. Isoleucine can bind to an allosteric site on the first enzyme in this pathway and function as a non-competitive inhibitor. As excess production of isoleucine inhibits further synthesis, it ensures stocks of threonine are not cannibalised in a cell (feedback inhibition).
MECHANISM-BASED INHIBITION
Mechanism-based inhibition involves molecules other than a substrate binding to an active site and being chemically altered. Covalent bonds form between the enzyme and substrate analogue, causing irreversible inhibition. An example of a mechanism-based inhibitor is penicillin, which inhibits specific transpeptidases from synthesising the bacterial cell wall. This prevents the bacteria from regulating the hydrostatic pressure within the cell, causing it to lyse. Penicillin is therefore a commonly used antibiotic, as it targets a feature unique to prokaryotic cells (peptidoglycan cell wall). However, certain strains of bacteria have mutations to their transpeptidase gene, resulting in low affinity for penicillin. These strains are resistant to treatments of penicillin and can potentially transfer this resistance to other strains
Cell Respiration
ENERGY CONVERSIONS
Organic molecules store energy in their chemical bonds – but this energy is not easily accessible for use by the cell. Cell respiration transfers this stored energy into coenzymes. Two types of coenzymes are used:
· ATP: Immediately available energy source (energy is released for use when ATP is hydrolysed to ADP)
· Hydrogen carriers: Transitional energy source (carries high energy electrons and protons for transfer)
ATP can be produced directly from organic molecules via substrate level phosphorylation (pink arrow) or it can be indirectly synthesised by hydrogen carriers (needs O2) via oxidative phosphorylation (yellow arrow).
TYPES OF CELL RESPIRATION
Cell respiration involves one of two reaction pathways – either anaerobic respiration or aerobic respiration.
ANAEROBIC RESPIRATION
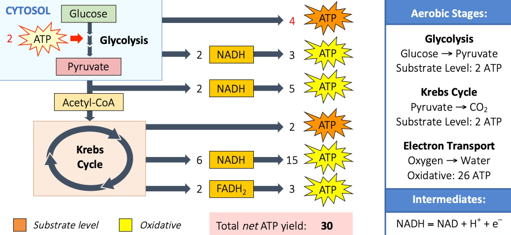
Anaerobic respiration involves the partial breakdown of carbohydrates (glucose) in the absence of oxygen. It occurs in the cytosol and results in a low yield of ATP (net production = 2 ATP). This ATP is produced via substrate level phosphorylation. The process of anaerobic respiration involves glycolysis and fermentation.
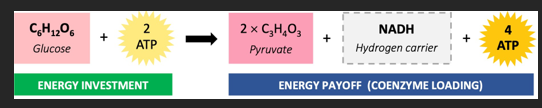
GLYCOLYSIS
Both anaerobic and aerobic respiration begins with the breakdown of glucose in the cytosol via glycolysis. Glycolysis splits glucose into two molecules of pyruvate in a process that consumes two molecules of ATP. However, four molecules of ATP are produced via substrate level phosphorylation, resulting in a net gain of two ATP molecules. Additionally, the coenzyme NAD is loaded with hydrogen to form molecules of NADH.
FERMENTATION
In the presence of oxygen, the hydrogen carriers produced by glycolysis may be used by the mitochondria to produce large amounts of ATP (via oxidative phosphorylation). However, in the absence of oxygen the hydrogen carriers must be unloaded to allow for glycolysis to continue (NADH must be unloaded to NAD). Fermentation involves the conversion of pyruvate via a reaction that unloads hydrogen carriers to restore stocks of NAD. In plants and yeasts, pyruvate is irreversibly converted into ethanol and carbon dioxide. In animals, pyruvate is converted into lactic acid (however, this reaction can be reversed if oxygen is present).

AEROBIC RESPIRATION
Aerobic respiration completes the breakdown of glucose begun by glycolysis. This process requires oxygen and occurs within the mitochondrion. The process of aerobic respiration involves three distinct reactions:
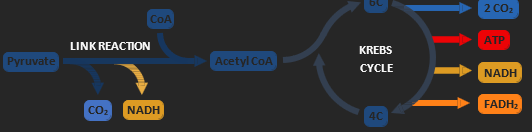
• Link Reaction: Pyruvate is shuttled into the mitochondria and converted into acetyl CoA (CO2 released)
• Krebs Cycle: Pyruvate is broken down to make carbon dioxide and large amounts of hydrogen carriers
• Electron Transport Chain: Hydrogen carriers are unloaded to produce ATP (oxidative phosphorylation)

MITOCHONDRIA
The mitochondrion is an organelle in eukaryotic cells that is responsible for aerobic respiration. It is believed to have evolved via endosymbiosis, when an aerobic bacterium was engulfed by another prokaryotic cell. Evidence for this endosymbiotic origin includes the fact that the mitochondrion possesses circular DNA, 70S ribosomes has a double membrane. In terms of structure, the central region is called the matrix and is the location of the Krebs cycle. Mitochondria contain an inner membrane that is highly folded into cristae. The cristae are the site of the electron transport chain. By folding the inner membrane, the SA:Vol ratio is increased, which optimises electron transport
LINK REACTION
The link reaction connects the products of glycolysis with the aerobic processes in mitochondria. Pyruvate is transported from the cytosol into the matrix by carrier proteins on the mitochondrial membrane. It then loses a carbon atom (to form carbon dioxide) and hydrogen atoms (NAD is reduced to NADH). The acetyl compound is then combined to coenzyme A to form a final compound of acetyl coenzyme A (acetyl CoA).
KREBS CYCLE
The acetyl CoA is combined with a 4C compound to make a 6C intermediate. This is then broken back down into the original 4C compound over a series of reactions collectively called the Krebs cycle (citric acid cycle). Each Krebs cycle produces carbon dioxide molecules, a small yield of ATP and a large quantity of hydrogen carriers (NADH and FADH2). These hydrogen carriers will then be unloaded via the electron transport chain.
ELECTRON TRANSPORT CHAIN
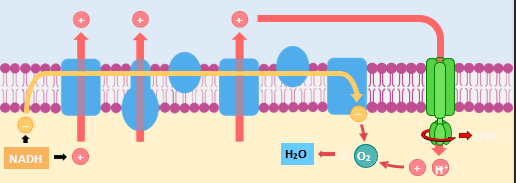
Hydrogen carriers (NADH + FADH2) are unloaded in the matrix to release protons and energised electrons. The electrons move across an electron transport chain on the inner membrane and become de-energised. The energy is used to pump protons into the intermembrane space, creating an electrochemical gradient. The protons diffuse back into the matrix via a transmembrane enzyme called ATP synthase, which uses the movement to catalyse ATP synthesis. The diffusion of protons down an electrochemical gradient is referred to as chemiosmosis. For the electron transport chain to continue to function, the de-energised electrons must be removed from the chain. Oxygen acts as the terminal electron acceptor, combining the electrons with protons in the matrix to form water. Because the synthesis of ATP involved the oxidation of hydrogen carriers, the ATP is produced via oxidative phosphorylation (as opposed to substrate level phosphorylation).
ENERGY SOURCES
Cell respiration involves the breakdown of organic compounds. Carbohydrates (glucose) are typically used as substrates for aerobic or anaerobic respiration because they are easy to digest and transport. Lipids are used as an alternative energy source, but can only be used aerobically – fatty acid chains are broken down into 2C compounds that form acetyl CoA (bypassing glycolysis). Lipids produce more energy per gram than carbohydrates as the carbon chains possess fewer oxygen but have more oxidizable hydrogen and carbon.
Photosynthesis (again)
CHLOROPLAST
The chloroplast is an organelle in the leaf tissue of plants that is responsible for photosynthesis. It is believed to have evolved via endosymbiosis, when a photosynthetic prokaryote (a cyanobacterium) was engulfed by another cell. This is evidenced by the presence of circular DNA (naked) and 70S ribosomes. In terms of structure, chloroplasts contain membrane discs called thylakoids that are arranged into stacks called grana. These thylakoids have chlorophyll
and are the site of the light dependent reactions. The fluid of the chloroplast is called the stroma and is the location of the light independent reactions.
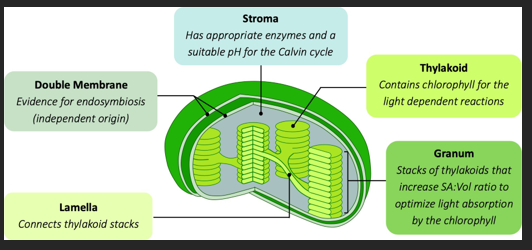
PHOTOSYSTEMS
Photosynthetic organisms do not rely on one pigment to absorb light, but instead benefit from the combined action of many. The pigments are grouped into molecular arrays called photosystems that are located within a thylakoid membrane. When a pigment is energised by light, it releases high energy electrons. Accessory pigments transfer their energised electrons to a central reaction centre containing a special chlorophyll. The release of energised electrons from the reaction centre allows chloroplast to produce ATP and reduce hydrogen carriers (NADP → NADPH). By grouping pigments into photosystems, the cell maximises light absorption.
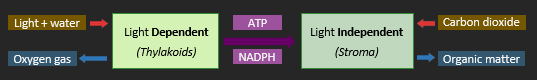
PHOTOSYNTHESIS
Photosynthesis is a two-step process whereby cells harness light energy to synthesise organic molecules. The light dependent reactions convert light energy into chemical energy (ATP) and splits water to release hydrogen atoms. The light independent reactions use the chemical energy to fuse the hydrogen atoms to a carbon source (CO2), producing organic compounds. The two photosynthetic reactions are interdependent (the light dependent stage loads the coenzymes while the light independent stage unloads the coenzymes).
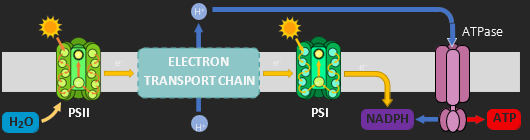
LIGHT DEPENDENT REACTIONS
The light dependent reactions consist of two photosystems within the thylakoid membrane. Photosystem II releases electrons into an electron transport chain. As energised electrons pass through the chain they lose energy, which is used to translocate protons from the stroma into the thylakoid lumen. The accumulation of protons in the lumen creates an electrochemical gradient. The protons return to the stroma via an enzyme within the membrane called ATP synthase (this proton movement is called chemiosmosis). ATP synthase uses the passage of protons to catalyse ATP synthesis (photophosphorylation). The de-energised electrons are taken up by a second photosystem. Photosystem I accepts the electrons from the transport chain as a replacement for the energised electrons it transferred to a hydrogen carrier (NADP → NADPH). The initial electrons from Photosystem II are replaced by the photolysis of water. Photosystem II contains an enzyme that is able to split water molecules into hydrogen (i.e. protons and electrons) and oxygen (a by-product).
The products of the light dependent stage (ATP and NADPH) are transferred to the light independent stage.
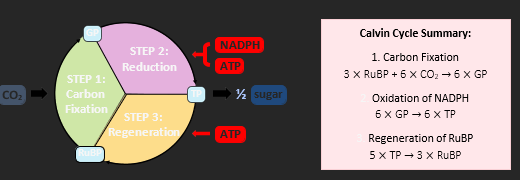
LIGHT INDEPENDENT REACTIONS
The light independent reactions use the chemical energy derived from light dependent reactions to form organic molecules. This is accomplished by a series of chemical reactions collectively called the Calvin cycle. In the Calvin cycle, an enzyme called Rubisco catalyses the attachment of carbon dioxide to a 5C compound called RuBP. The resulting 6C compound is unstable and is broken down into two 3C compounds called GP (glycerate-3-phosphate). The GP is next converted into a triose phosphate (TP), using protons and electrons from NADPH and energy from ATP. Some of the TP produced is then used to form organic compounds, the remainder is used to regenerate stocks of RuBP (completing the cycle). The regeneration of RuBP requires ATP hydrolysis. A single cycle produces one molecule of TP – two cycles are required to synthesise glucose.
ORGANIC COMPOUNDS
The triose phosphate produced by the Calvin cycle is used to synthesise a variety of organic compounds. The four main types of organic compounds are carbohydrates, lipids, nucleic acids and proteins. Synthesis of these molecules involves a variety of metabolic reactions that take place within the cytoplasm. Some of these organic molecules may require the additional incorporation of mineral ions. Proteins and nucleotides both require nitrogen, while nucleic acids and phospholipids require phosphorus. Certain amino acids also need sulphur. Organic matter made by photosynthetic organisms is ingested and assimilated by consumers.
OVERVIEW OF PHOTOSYNTHESIS