Bonding
Drawing Lewis structures:
Atoms and ions are most stable when they are isoelectronic to noble gases (noble gas-like structures)
Electrons are most stable when paired
Atoms form bonds to have a full valence shell
Full valence shell can be achieved by the sharing of electrons in nonmetals covalent bonds
Full valence shell can be achieved by the transfer of electrons in metals and non-metals ionic bonds
Lewis structures show the arrangements of electrons and bonds in a molecule or polyatomic ion.
Hydrocarbons with double or triple bonds react more quickly than hydrocarbons with single bonds.
Double/ triple are shorter and stronger than single bonds
Steps for drawing Lewis structures:
Identify the central atom, the atom with the highest bonding capacity ( lowest electronegativity)
Add up the number of valence electrons in each present atom - give the total number of dots needed -
if polyatomic ion, add/subtract electrons depending on the charge (add electrons if the charge is negative)
Start to bond the peripheral atom to the central atom (one dash = 2 electrons) and add lone pairs on the peripheral atoms
Place the remaining electrons on the central atom and check for the formal charge (if the formal charge = 0, it’s good)
formal charge = valence electron of the neutral atom - number of valence electrons around the atom
Add double bonds to atoms with a formal charge higher than the expected formal charge, and remove double bonds from atoms with a formal charge lower than the expected formal charge.
VSEPR theory:
The Valence Shell Electron- Pair Repulsion Theory is a method to determine the geometry of a molecule based on the idea that electrons want to stay as far away from each other
Electrons are negatively charged, thus repel each other
Bonded electrons and lone pairs position themselves as far away from each other to minimize the repulsive force between them.
Lone pairs decrease the bond angles between the bonded atoms.
There are no bond angles between the bonded atoms and lone pairs
Double/triple bonds do not affect the bond angles but instead bring resonance to the molecule (the idea that atoms take turns with double bonds as a way to stabilize the molecule)

Electronic geometry is the shape of the bonded atoms + lone pairs
Molecular geometry is the shape of only the bonded atoms
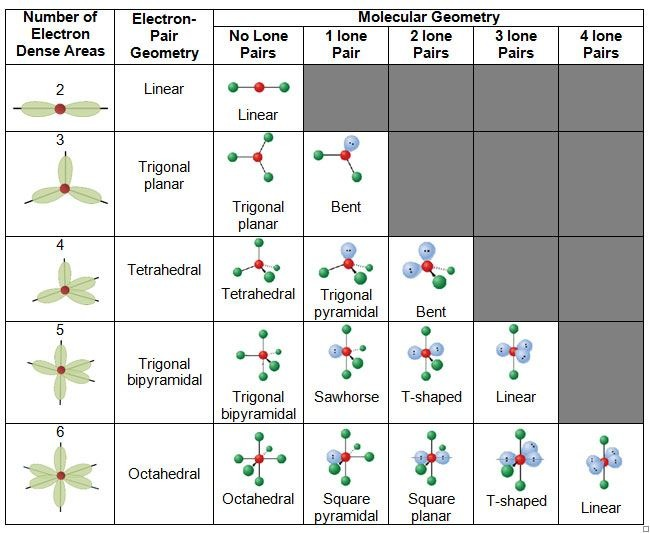
When there is more than one central atom, predict the electron arrangement around each atom individually. Then combine these arrangements to determine the overall geometry.
Valence bond Theory
States that atoms form overlapping orbitals with a pair of opposite spin electrons when bonding
Covalent bonds are formed from the overlap of atomic orbitals
Atomic orbital is a region where electrons are most likely to be found
If two atoms are approaching each other and their orbitals are in phase, they would overlap constructively and create a bond (covalent)
If those two atoms approche each other and their orbitals are out of phase, there would be no bond, and instead a node. (Zero electron density)
Covalent bonds is the sharing of electron density between two atoms as a result of constructive interference between the atomic orbitals.
Hybrid orbitals are used to form sigma bonds (single bonds)
Unhyberdized are used to form pi bonds (double/triple bonds)
When two orbitals overlap head to head, its called a sigma bond (produces lowest energy bonds (most stable), best attraction)
All single bonds are sigma bonds
Linear = Sp hybrid orbitals - right in between s and p
Trigonal planter = sp2 hybrid orbitals - between s and p, but a bit closer to p
Tetrahedral = sp3 hybrid orbitals - closer to p
Trig bipy = sp3d hybrid orbitals - in between p and d, but closer to p
Octahedral = sp3d2 hybrid orbitals - in between p and d
When there is a filled orbital in the final hybridized state, it is a lone pair
In a multiple bond, the orbitals that are parallel overlap and form pi bonds
A double bond consists of one pi bond and one sigma bond
A triple bond consists of two pi bonds and one sigma bond
Polarity
Polarity is only a matter of covalent bonds, not ionic bonds
To look if a bond is polar or non polar, look at the electronegativity values
EN < 0.5 = Non polar
EN > 0.5 =Polar
To represent the dipole moment, draw an arrow that starts from the last electronegative side (delta +) and points to the highest electronegative side (delta -)
If all the dipole moment cancel (equal length/force but facing opposite direction), the molecule is non polar.