Untitled Flashcards Set
Moles, Mass, & Atoms
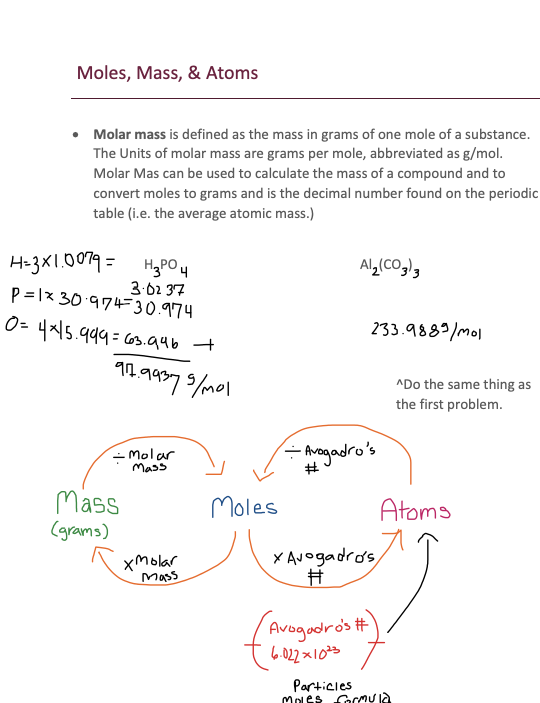
· Molar mass is defined as the mass in grams of one mole of a substance. The Units of molar mass are grams per mole, abbreviated as g/mol. Molar Mas can be used to calculate the mass of a compound and to convert moles to grams and is the decimal number found on the periodic table (i.e. the average atomic mass.)
H PO Al (CO )
^Do the same thing as the first problem.
Percent Composition:
· Percent composition may be calculated from experimental data or from the atomic mass (molar mass) of the compound, using the following equation:
· Percent (%) Comp. =
mass of element in compound/total mass of compound or molar mass x 100
1. Calculate the percent composition of each element when it is found that 1.58 g Cu reacts with sulfur to form 1.98 g of the compound.
Cu= (1.58/1.98) x 100= 79.8% [made up of copper]
S=100-79.8= 20.2%
(0.40/1.98) x 100
2. Determine the percent composition of the element that makes up magnesium phosphate.
Mg3(PO4)2 ---> Mg= 3 x 24.305= 72.915
P= 2 x 30.974= 61.948
O= 8 x 15.999= 127.992 +
--------------------
262.855 g/mol
Mg= 72.915/262.855= 27.7%
P= 61.948/262.855= 23.6%
O= 127.992/262.855= 48.7%
Stoichiometry:
· Involves mass/mole relationships between reactants and products in a chemical reaction.
· Stoichiometry is the areas of chemistry in which calculations of quantities in a chemical reaction are made.
i. You must have a balanced chemical equation.
ii. The coefficients in the equation are written in terms of moles. The relationship between these coefficients is called mole rations.
Example:
Sn (s) + 2 HF (g) SnF (s) + H (g)
o How many moles of hydrogen fluoride are needed to produce 2.5 moles of tin (2) fluoride
2.5 mole SnF x (2mol HF/1mol SnF)= 5.0mol HF
o What mass of tin is needed to produce 20.0 g of tine (2) fluoride?
20.0 g SnF x (1mol/156.706g) (1mol Sn/ 1mol SnF )
Divide the molar mass of the element/compound given to you
Mole Ratio
(118.71g/ 1mol)= 15.2 g Sn
X molar mass of what you are solving for
o Phosphorous (P ) burns in oxygen to form solid tetraphosphorous decoxide will be produced from 35.12 g of phosphorous?
P + 5O P O
Why do reactions stop?
· The limiting reactant limits the extent of the reaction and determines the amount of product formed.
· Reactants leftover when a reaction stops are excess reactants.
Solving limiting reactant problems:
i. Balance the chemical reaction given off if not already done.
ii. Use stoichiometry for each individual reactant to find the mass of product produced.
a. You will be provided a mass for each reactant.
b. IF multiple products are present convert each reactant to THE SAME product.
iii. The reactant that produces a lesser amount of product is the limiting reagent… the lesser amount of product is also the maximum amount that can be produced.
Example:
o What mass of water can be made from 3.75gH and 25.0gO
2H (g)+O (g) 2H O(g)
The lesser answer is the right answer of the two
LR is the smaller one
ER is the larger one
o If 35.4g CO and 11.5g H , are combined to give the reaction below…
CO + 2H CH OH
a. Identify the limiting reactant.
b. How many grams of CH OH could be produced?
LR=CO ER=H
c. How much excess reactant remains?
…mass-to-mass conversion from the LR (original amount) to ER
35.4gCO(1mol/28.01molCO)(2molH,2/1molCO)(2.0158g/1mol)=5.10 H,2
[used]
11.5-5.10=6.40gH,2 [used]
o Nitogen gas can be prepared by passing gaseous ammonia (18.1g) over solid copper (2) oxide (90.4g) at high temperatures
2 NH,3 + 3 CuO _ N,2 + 3 Cu + 3 H,2O
%yield=(actual yield/ Theoretical Yield)x100
We will be given actual yield our answer is theoretical yield (the lesser answer)
 Knowt
Knowt