Fundamental Particles
Atomic Structure
Protons, Neutrons and Electrons
Protons, neurons and electrons are classed as sub-atomic particles
Atomic number = number of protons in an atom
Mass number = number of protons and neutrons inside the nucleus of an atom
Number of neutrons = Mass number - atomic number
Positive Ions = Have more protons than electrons
Negative Ions = Have more electrons than protons
The Atomic Model
J.J Thomson’s Model of the Atom (Plum Pudding Model)
His model had these features:
Negatively shaped particles called electrons randomly spread in the atom
Atom is mostly a large positive ball
The atom is solid throughout
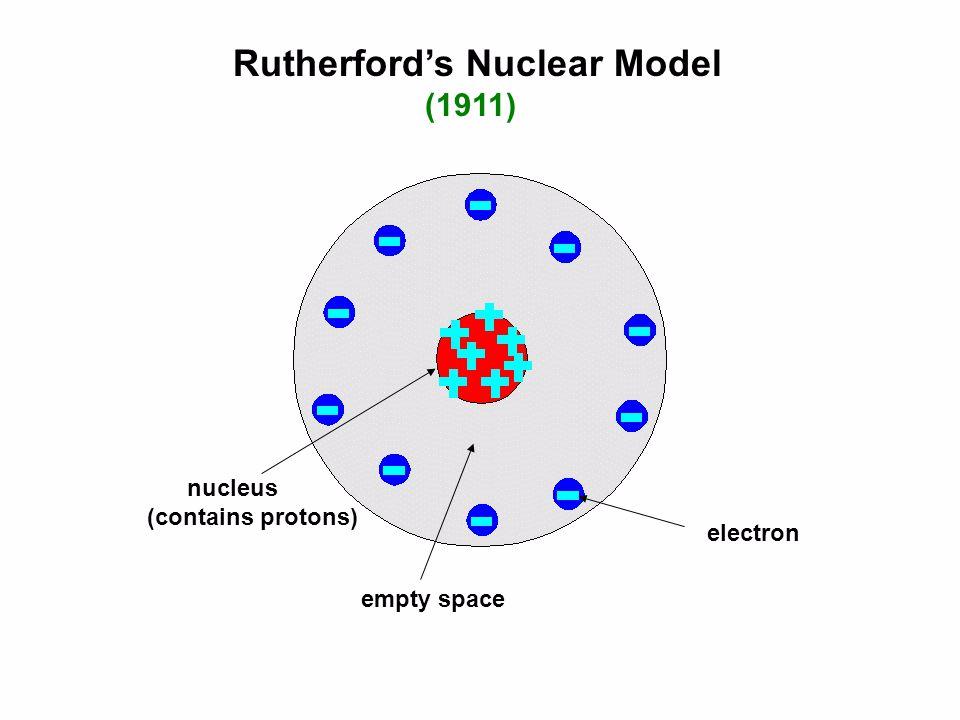
Rutherford’s Model of the Atom (Nuclear Model)
His model had these features:
Nucleus:
Rutherford proposed that an atom consists of a tiny, dense, positively charged nucleus at the center. Most of the mass of the atom is concentrated in this nucleus, which is composed of protons (and, as later discovered, neutrons).
Electron Cloud:
Electrons move in regions of space around the nucleus, which is often referred to as the "electron cloud." The electrons are in constant motion, and their paths are not well-defined. This implies that atoms have a lot of empty space.
Nuclear Structure:
The nucleus contains protons, which are positively charged, and is later found to also include neutrons, which have no charge. The presence of protons in the nucleus gives the nucleus its positive charge.
Size of the Atom:
The nucleus is extremely small compared to the overall size of the atom. For example, if the atom were the size of a football stadium, the nucleus would be the size of a small marble.
Electrostatic Forces:
Rutherford proposed that the electrons are held in orbit around the nucleus by electrostatic forces. The negative charge of the electrons attracts them to the positively charged nucleus.
Gold Foil Experiment:
The model was based on observations from Rutherford's famous gold foil experiment, where alpha particles were directed at a thin sheet of gold foil. Most particles passed through, but some were deflected at large angles. This suggested that a small, dense nucleus exists to account for the deflections.
Limitations:
While Rutherford's model successfully described the nuclear structure of the atom, it could not accurately explain certain phenomena, such as the stability of electron orbits or the discrete spectra of elements. These limitations would later be addressed by the development of quantum mechanics and the Bohr model of the atom.
Size of an Atom
Average radius of an atom is 0.1nm (1×10-10m)
Average radius of the nucleus is 10fm ((1×10-14m)
Bohr’s Model of the Atom
Quantized Energy Levels:
Bohr proposed that electrons orbit the nucleus in fixed paths or "shells," each corresponding to a specific energy level. Unlike classical physics, where an electron could theoretically occupy any energy level, Bohr's model introduced the idea that only certain discrete energy levels are allowed.
Stationary States:
Electrons in these orbits, or stationary states, do not emit radiation or lose energy. They can remain in these stable orbits without spiraling into the nucleus, as was a concern in Rutherford's model.
Time-of-Flight Mass Spectrometry (TOF)
Isotopes
Isotopes are atoms of the same element, but with the same number of protons a different number of neutrons.
Isotopes of the same element have the same chemical properties as they have the same electron configuration.
Electrospray Ionisation
Formation of Charged Droplets: ESI involves the use of a high-voltage source to create a fine aerosol of charged droplets from a liquid sample. The sample solution is pumped through a narrow capillary that has a high voltage applied to it
Protonation: In the ESI process, analytes often gain protons (H⁺), resulting in the formation of positively charged ions (cationic species).
Acceleration
After ionization, these ions need to be accelerated so they can travel through the mass spectrometer.
A high voltage is applied between two electrodes (like a battery). One electrode is at the ion source where ions are produced, and the other is a nearby plate.
When the voltage is applied, it creates an electric field. This field pushes the ions away from the source, speeding them up.
Drift (Flight Tube)
The ions leave the electric field with a constant speed and constant kinetic energy. They enter a region with no electric field and drift through it at the same speed they left the electric field. So ions with a lower m/z ratio will be drifting at higher speeds.
Time of flight along a flight tube is given by this equation: T = d / v, where
T = time taken
d = distance travelled
v = velocity of ion
Constant KE (Kinetic Energy) by an electric field towards a negatively charged plate. Lighter ions travel faster since their speed is dependent on their mass.
Detection
When ions hit the detector, a few things happen
Electrons flow from negative plate (the detector) to the positive ions
A current is induced
Detector will record how long it took the ions to pass through the spectrometer
Calculate m/z ratio of the particular ion.
The greater the number of ions that hit the detector at a given time, the greater the current that will be induced, and therefore indicate a greater abundance of that ion.
Time of Flight Calculations

Mass Spectra
Understanding a Mass Spectrum:
A mass spectrum typically consists of the following features:
X-axis (m/z): Represents the mass-to-charge ratio of the ions. Is typically plotted on a linear scale.
Y-axis (intensity): Represents the relative abundance or intensity of the detected ions. It can be plotted on a linear or logarithmic scale.
Peaks: Each peak corresponds to a specific ion or a set of ions with similar m/z values. The height or area of the peak indicates the relative abundance of that particular ion.
Analyzing Mass Spectra:
Identifying Peaks: Each peak can correspond to a molecular ion (M⁺) or fragment ions generated from the ionization process.
Isotopic Patterns: Analyzing the isotopic distribution can help deduce the elemental composition of the molecule.
Fragmentation Patterns: Understanding how a molecule breaks apart can reveal structural information.
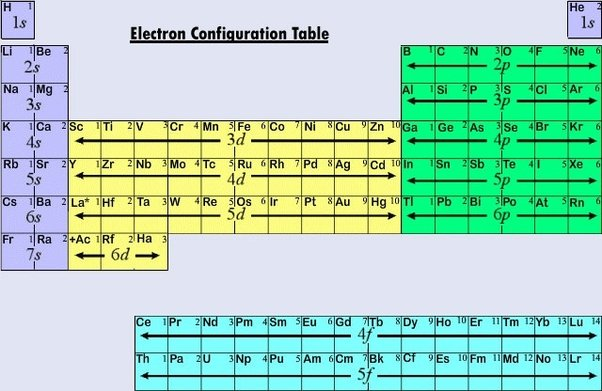
Electron Configuration
Electron Arrangement
Orbitals
There are 4 orbitals:
s orbitals: Spherical; closest to the nucleus
p orbitals: Dumb bell shaped
d orbitals: Clover shaped
f orbitals: Clover-clover shaped

Exceptions to the Rule:
Chromium (Cr): 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5
This is because this allows the electrons in the 4s and 3d subshells to fill in singly, and is therefore more stable.
The reason for this deviation is that having a half-filled 3d subshell (with 5 electrons) provides greater stability due to reduced electron-electron repulsion and enhanced exchange energy.
Copper (Cu): 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d10
This is because it allows all orbitals in the 3d orbital to be filled and creates a more stable atom.
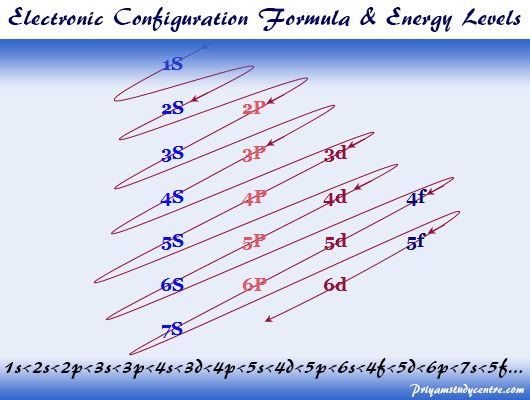
Electron Filling Order
Electrons enter the lowest energy orbital available
Orbitals of equivalent energy are filled singularly before pairing occurs
Each orbital can take a maximum of 2 electrons
Ionisation Energy Data
Ionisation Energy
Ionisation Energy: Amount of energy needed to remove an electron from a gaseous atom to produce a gaseous ion. The electron is said to be removed to infinity
A large change in IE happens when an electron is removed from a energy level (shell) closer to the nucleus (Group 0 electron → Group 7 electron)
1st Ionisation Energy = X(g) → X+ + e-
2nd Ionisation Energy = X+(g) → X2+ + e-
3rd Ionisation Energy = X2+(g) → X3+ + e-
4th Ionisation Energy = X3+(g) → X4+ + e-
Trend in Ionisation Energies down ALL groups
Down the group:
There is a decrease in ionisation energy
As the energy removed is in an energy level at a greater distance away from the nucleus
Therefore the electron becomes the successively easier to remove as it is more shielded and not as firmly held by the nucleus
Weaker electrostatic attraction between Nucleus and Outer Electron
Trend in Ionisation Energies across Period 3
Ne → Na (Any group 0 → group 1):
Large Decrease in 1st Ionisation Energy (IE) from Ne → Na
In Na, outer electron is lost from an energy level further from the nucleus than Na
There is more shielding in Na than Ne
Less attraction from nucleus to outer electron, so less energy required to remove it
Na → Mg (Any group 1 → group 2 ):
Small increase in 1st IE from Na → Mg
Mg has 1 more proton in the nucleus than Na
Outer electron in same energy level and subshell; similar/same shielding
Greater attraction from nucleus to outer electron; more energy required to remove it
Mg → Al (Any group 2 → group 3):
Despite nuclear charge increasing and shielding increasing,
Small decrease in 1st IE from Mg → Al
In Al, outer electron is lost from a subshell further from the nucleus (of higher energy) [3p instead of 3s]
So less attraction from nucleus to outer electron and therefore less energy required to remove it
Al →Si → P (Any group 3 → group 4 → group 5):
Increase in 1st IE from Al → Si → P
Due to increased nuclear charge (higher number of protons in nucleus)
Outer electrons in same energy level and subshell so similar shielding
Greater attraction from nucleus to outer electron; more energy required to remove it
P → S (Any group 5 → group 6):
Despite nuclear charge increasing, and despite being in the same subshell,
Small decrease in 1st IE from P → S
Due to pairing in the p-subshell for the first time
This causes repulsion between electrons, lowering the ionisation energy needed to remove an electron
S → Cl → Ar (Any group 3 → group 7 → group 8):
Increase in 1st IE from S → Cl → Ar
Due to increase in nuclear charge
Outer electron is in the same energy level and subshell, so similar shielding
More energy required to remove the electron
Ar → K:
Same as Ne → Na
SANE
SANE stands for:
S = Same (orbital) / shielding
A = Atomic radius
N = Nuclear charge (number of protons)
E = Electron repulsion between electrons