L13: Fluorescence quenching
Fluorescence quenching refers to any process that decreases the fluorescence intensity of a sample.
Common Molecular Interactions Leading to Quenching:
Excited-state reactions
Molecular rearrangements
Energy transfer
Ground-state complex formation
Collisional quenching
Stern-Volmer plot and its application
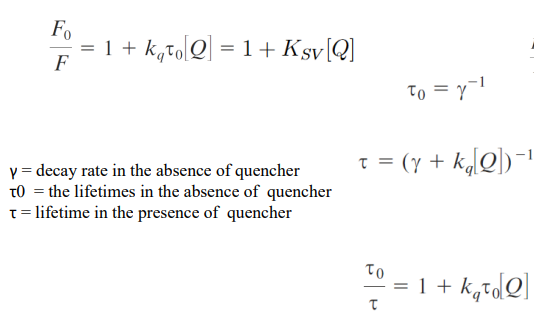
intensity is proportional to lifetime
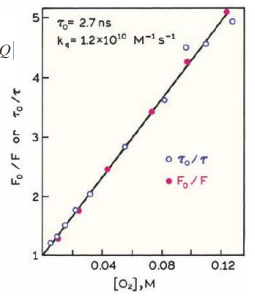

Connecting quenching rates to collision
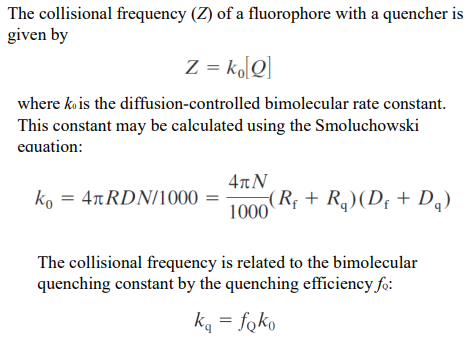
Smoluchowski equation
Static quenching
Static quenching occurs when a nonfluorescent ground-state complex forms between the fluorophore and quencher.

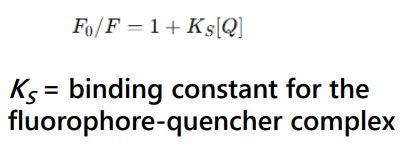
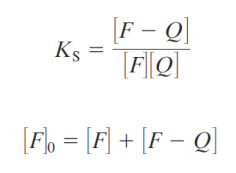
Stacking interactions between purine and pyrimidine nucleotides and some fluorophores are common examples of static quenching.
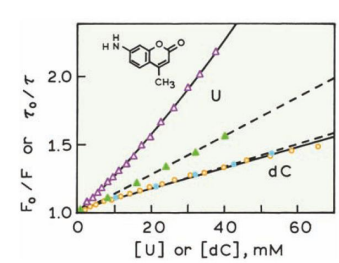

The intensity Stern-Volmer plot (open triangles) shows upward curvature.
The lifetime Stern-Volmer plot (solid triangles) is linear and shows less quenching than the intensity data.
The greater quenching seen in the intensity plot compared to the lifetime plot indicates that quenching is driven by:
Complex formation (static quenching)
Collisional interactions (dynamic quenching)
Conclusion: Uridine quenches C-120 through both static and dynamic quenching mechanisms.
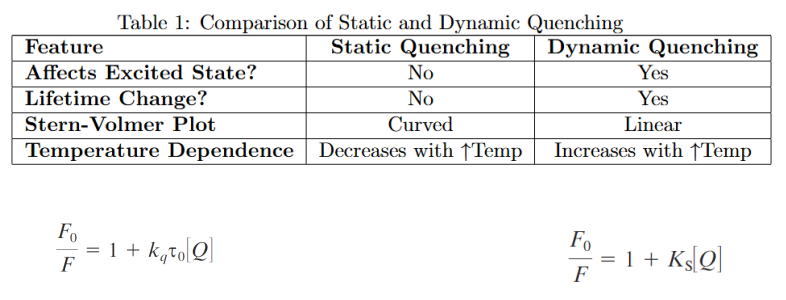
Static vs dynamic quenching
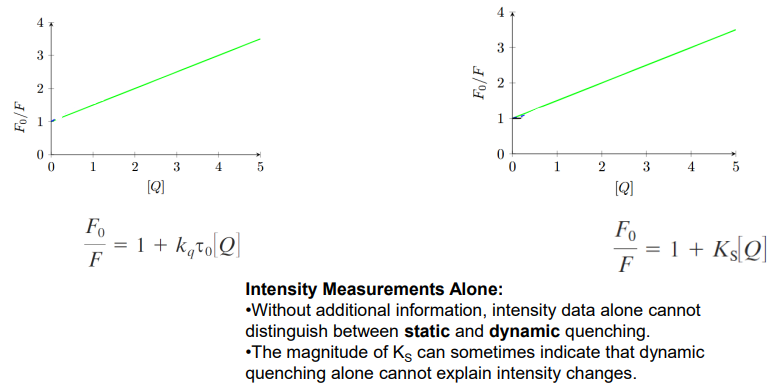
Intensity Measurements Alone:
Without additional information, intensity data alone cannot distinguish between static and dynamic quenching.
The magnitude of KS can sometimes indicate that dynamic quenching alone cannot explain intensity changes.
Fluorescence Lifetime Measurement (Most Reliable Method):
Static Quenching:
Removes a fraction of fluorophores by forming non-fluorescent complexes.
Only uncomplexed fluorophores emit fluorescence.
Lifetime remains unchanged → τ0/τ=1
Dynamic Quenching:
Quenching occurs via excited-state collisions.
Lifetime decreases → F0/F=τ0/τ
Absorption Spectra Analysis:
Dynamic Quenching: No change in the absorption spectrum (affects excited state only).
Static Quenching: Often causes perturbations in the absorption spectrum due to ground-state complex formation.
Static quenching can modify the extinction coefficients of the free and complexed fluorophore forms, making absorption data a useful tool for identification.
Combined static and dynamic quenching
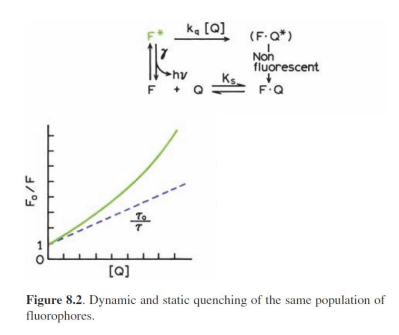
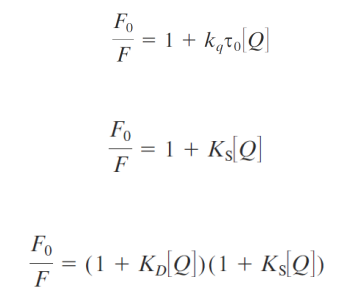
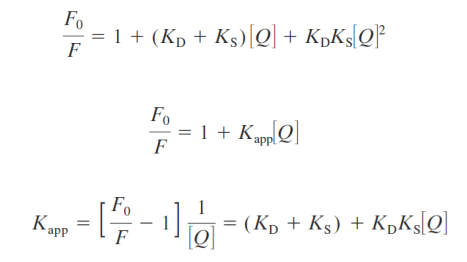
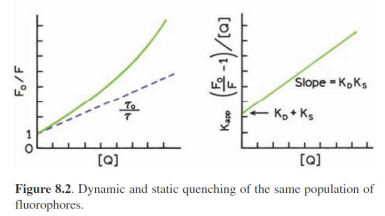
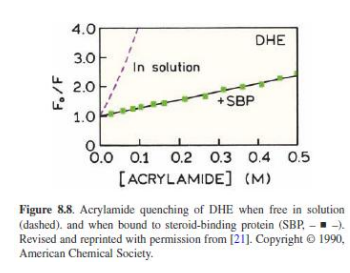
Accessibility of macromolecule-Bound Probes to Quenchers
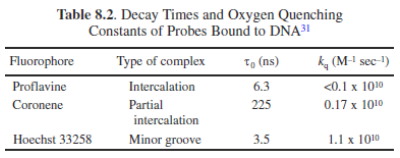
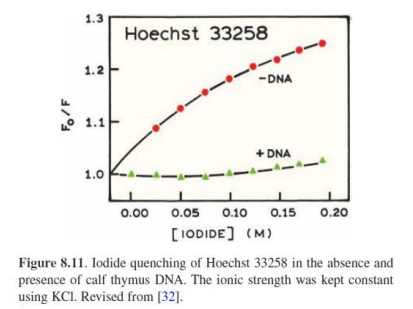
Possible mechanism: Negative charges on DNA prevent iodide from coming into contact with Hoechst 33258 when bound to the minor groove of DNA
Applications
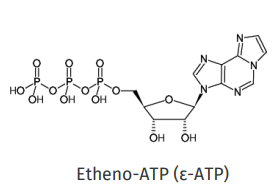
Quenching of Ethenoadenine Derivatives
DNA nucleotide bases are generally nonfluorescent.
•A fluorescent analogue of adenine, ε-ATP, is created by adding an etheno bridge.
The charge on ε-Ad nucleotides varies with pH and phosphorylation:
ε-ATP: Charge = −3
Ethenoadenosine: Charge = 0
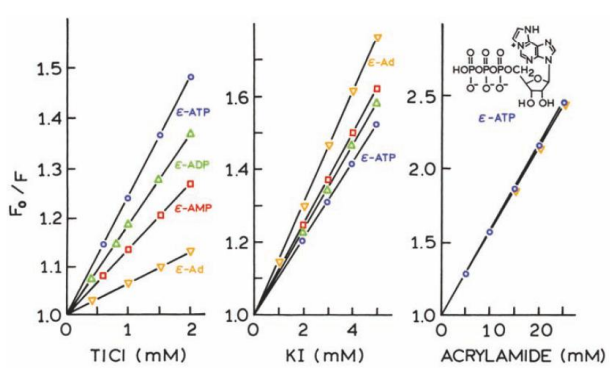
Acrylamide (Neutral Quencher):
No dependence on nucleotide charge — consistent Stern-Volmer plot.
Tl+ (Positively Charged Quencher):
Maximum quenching observed for ε-ATP (most negative charge).
Quenching decreases as phosphate groups are removed.
Iodide (Negatively Charged Quencher):
Least quenching observed for ε-ATP (most negative charge).
Quenching increases as the negative charge is reduced.
1. Charge interactions play a significant role in quenching efficiency.
2. This method can reveal the local charge environment (Å/sub-Å) around fluorophores in macromolecules by testing quenching effects with differently charged quenchers.
Review
Reading for today’s lecture Lakowicz: Ch 1, 6.1, 6.4, 8.1, 8.2, 8.8, 8.9
Fluorescence quenching provides insights into molecular interactions and fluorophore accessibility.
Combining Stern-Volmer analysis with lifetime and absorption data allows precise identification of quenching mechanisms.
Charge-based quenching strategies offer powerful tools for probing biomolecular structure and dynamics.
Interpreting Stern-Volmer Plots:
1. What features in a Stern-Volmer plot can help distinguish static from dynamic quenching?
2. How would you identify a system with combined static and dynamic quenching?
Molecular Environment Insight:
1. Why is charge an important factor in determining quenching behavior in biological systems?
2. How might quenching studies provide insights into protein conformational changes?
Experimental Considerations:
1. If a fluorophore shows no change in fluorescence lifetime but a drop in intensity, what steps would you take to confirm if the quenching is static?
(temperature, lifetime, inner filter effect, controls)
Quenching in Practice:
1. Imagine you are designing an experiment to probe DNA-protein interactions using fluorescence quenching. Which types of quenchers (positive, neutral, or negative) would you test and why?