Lab 3 - Freezing Point Depression
4 Colligative Properties
Osmotic pressure
Lowering of vapor pressure
Depression of freezing point
Elevation of boiling point
Freezing Point Depression
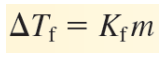
ΔT = difference between the initial freezing point of the pure solvent and the final freezing point
Kf = freezing point depression constant for the solvent (°C/m)
m = concentration (mol solute/kg of solvent) of the solution in molality
Molarity (M) & Molality (m)
Molarity = (moles of solute)/(liters of solution)
Molality = (moles of solute)/(mass of solvent in kg)
Molality is the number of moles of solute dissolved in 1 kg (1000g) of solvent
What advantage does Molarity have over Molality?
Molarity is great for measuring exact volumes
What advantage does Molality have over Molarity?
Molality is temperature independent (i.e. concentration does not change with temp.)
Freezing Point Depression Non-Electrolytes
Non-electrolytes do not dissociate into “pieces” of the formula units.
Ex: Sugar (a non-electrolyte) molecules will dissolve in water but will not come apart into many pieces in water. Therefore, one mole of sugar molecules in a solution will yield one mole of dissolved sugar molecules.
Van’t Hoff Factor (i)
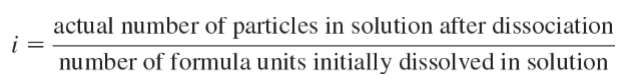 Electrolyte Solutions
Electrolyte Solutions
Using the van’t Hoff factor in the calculation accounts for the multiple moles of ions that go into solution when ionic compounds dissociate into their constituent cations and anions
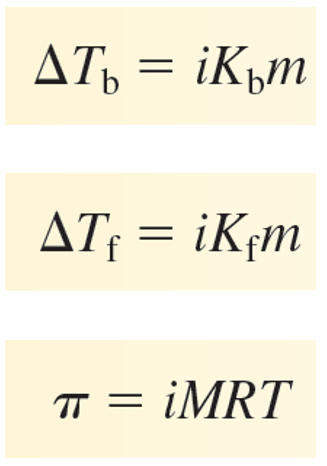
Effect of Adding a Solute
Decrease the solvent’s freezing point.
Increase the solvent’s boiling point
The degree of freezing point depression is related to the identify of the solvent, the molar mass of the solute, and the mass of the solution. This relationship is expressed as: 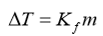
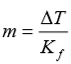
ΔT = difference between the initial freezing point of the pure solvent and the final freezing point
Kf = freezing point depression constant for the solvent (-1.86°C/m)
m = molal concentration (mol solute/kg of solvent) of the solution
2nd formula allows the determination of the number of moles of solute present
Molar mass = number of grams of solid initially weighed / number of moles of solute determined in the experiment