week 6; ADHD; symptoms and consequences
Characteristics of ADHD
Consists of hyperactivity, impulsivity and attentional problems
DSM-5: ADHD is ‘a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development’
often has difficulty sustaining attention in tasks or play activities
often fidgets with or taps hands or feet or squirms in seat
recognises three ‘presentations’: predominantly inattentive, predominantly hyper-active-impulses, combined
several symptoms have to be present before age 12 and manifest in more than one-context
ICD-11: ADHD (2010)- ‘ persistent patterns of inattentive and/or hyperactivity-impulsivity that has direct negative impact on functioning’
statistics of ADHD
UK - currently 5%
ratio of boys:girls = 3:1 - the manifestation is different, so this ratio could actually just be referencing the rate of diagnosis rather than actually stating that it is more common in boys
symptoms continue into adulthood in 8-43% of cases (gender gap may narrow)
activity issues decline, attentional issues remain
a frontline treatment (children over 5/ adolescents and adults): DL-amphetamine or methylphenidate (NICE guidelines)
estimated medical and non-medical cost (children/ adolescent; UK) - £2.4 billion
The history of ADHD
in 1902, George Fredric Still (paediatrician)
Described 20 cases of children with a “defect of moral control…. without general impairment of intellect and without physical disease”
moral control- they didn’t behave in a way of the good for everybody but rather for the good of themselves
“the immediate gratification of self without regard either to the good of others or to the larger and more remote good of self”
“fidgety….”
“a quite abnormal incapacity for sustained attention”
1978-28, Encephalitis Iethargica epidemic
Many affected children who survived the encephalitis, subsequently showed abnormal behaviour - “Postencephalitic behaviour disorder”
Children often became hyperactive, distractible, irritable, antisocial, destructive, unruly, and unmanageable in school
Because these symptoms came from this disease, it lead to the first association of ADHD characteristics with brain damage….
1940’s/ 50’s, minimal brain damage
Assumption that minimal damage to the brain, even when it cannot be demonstrated objectively, causes postencephalitic-type behaviour disorder
professionals started diagnosing those with these characteristics with brain damage
1960’s, minimal brain dysfunction (MBD)
Became clear that this disorder (“hyperkinetic impulse disorder”) could occur in the absence of explicit brain damage
The Oxford International Study Group of Child Neurology therefore advocated a shift in terminology by replacing the term “minimal brain damage” by “minimal brain dysfunction”
moving away a bit from the association of the characteristics automatically comes from brain damage
1968, DSM II “hyperkinetic reaction of childhood”
MBD was eventually considered too broad, leading to a focus on more specific and key symptoms… (especially hyperactivity)
1980, DSM III “attention deficit disorder (ADD) (with or without hyperactivity)”
Throughout 70s, developing realisation that attentional problems were as significant, or more significant, than hyperactivity in this patient group
Led to 1980 DSM III “Attention Deficit Disorder (ADD)”
could occur in two different ways and was the first in the DSM to address that the disorder was attention-based
Hyperactivity was no longer an essential diagnostic criterion for the disorder
Developed three separate symptom lists for inattention, impulsivity, and hyperactivity
1987, 1987 DSM III-R “Attention Deficit Hyperactivity Disorder”
Brought the symptom lists together, which is why it was labelled ADHD rather than ADD
The symptom list was separated again (3 subtypes) in 1994
1994-2013
1994 DSM IV, 2000 DSM-IV-TR and 2013 DSM 5
Dr Charles Bradley is credited with the discovery in the 1930s that amphetamine is efficacious in the treatment of ADHD whilst he was using it to stimulate the production of spinal fluid
Impact of ADHD
DSM 5: There is clear evidence that the symptoms interfere with, or reduce the quality of, social, academic, or occupational functioning
DSM 5: There is clear evidence that the symptoms interfere with, or reduce the quality of, social, academic, or occupational functioning
ADHD associated with:
Poorer educational attainment:
Fleming et al. (2017)
4 Scotland-wide NHS databases
4 Scotland-wide school databases
School leavers 2009-13
ADHD vs non-ADHD
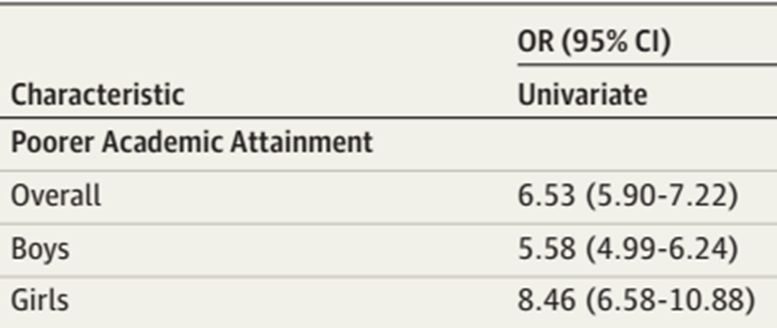
Likelihood (OR) of leaving school with qualifications below GCSE level depending on if you were a school member with ADHD or not
Sub-GCSE qualifications, they found that if you had ADHD you were 6.53% more likely to leave school with lower than GCSE level qualifications
Poor occupational attainment:
Klein et al. (2012)
adults (mean age 41) diagnosed with ADHD at 6-12 years of age (vs control), if the occupational level was different depending on if they had ADHD diagnosed as children or not


the smaller the number the higher up in the occupational chain you are, the occupational level was lower for those with ADHD, they were lower in the occupational ladder too and earned less
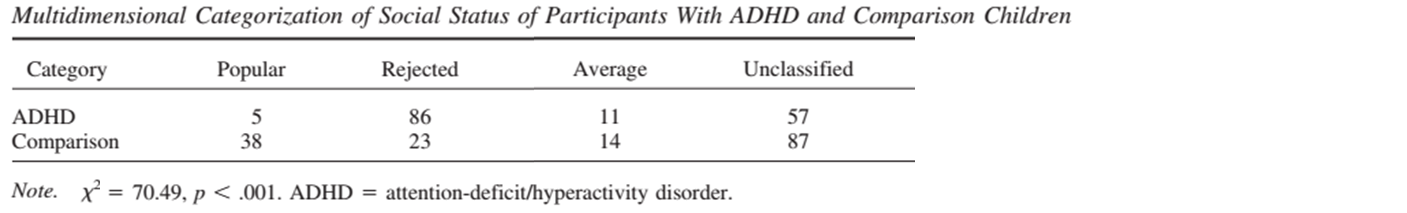
Problem with peer relations
Hoza et al. (2005)
Children with ADHD (7-9.9 years of age) and same sex classmates
Physical health problems
Lange et al. (2016)
accidents over the last 12 months requiring medical treatment in children with ADHD (10.4 years old) and controls)
ADHD Control
23.0% 15.3%
OR for accidents was 1.60 (95% confidence interval [CI] = 1.34-1.91)
likelihood of being injured is about 1.6% higher for those with ADHD
mental health problems
Larson et al., 2010
children 6-17, 60,000 children, 5000 of which had ADHD
looked at mental health problems in these children
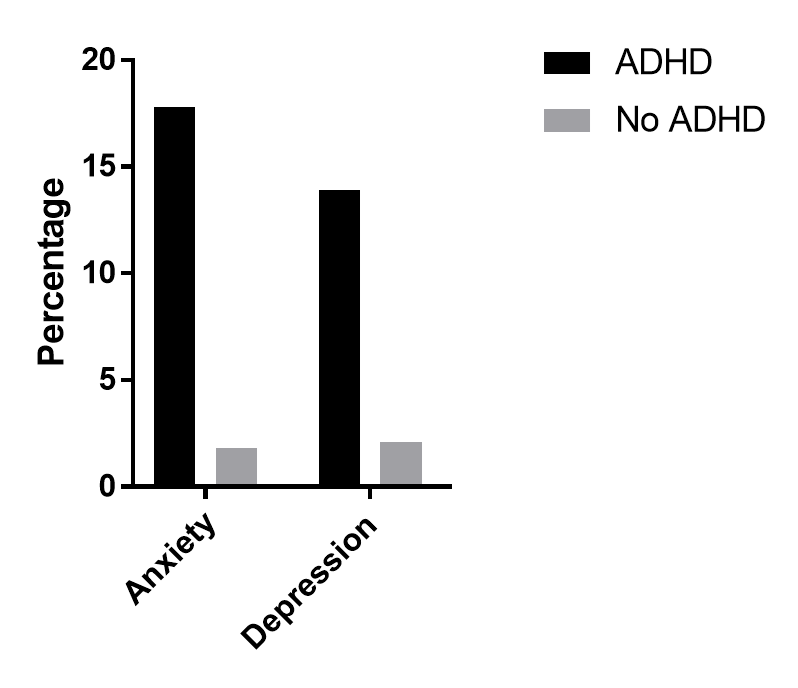
Anxiety and depression are the highest co-morbidity conditions for those with ADHD, but also even ASD and tourettes were more common for those with ADHD
Drug addiction
Levy et al., 2014
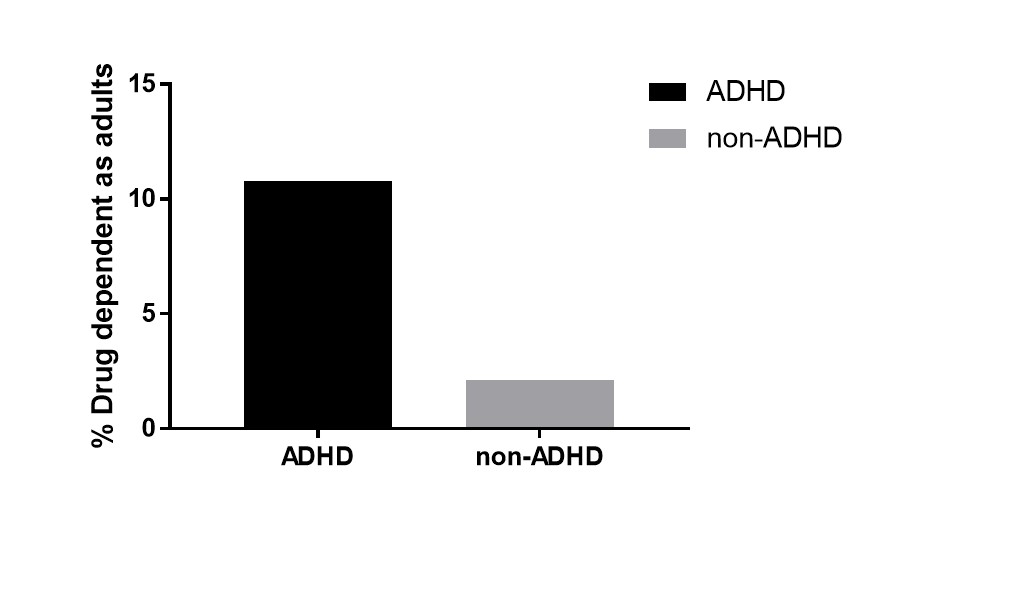
their non-alcohol drug dependency for those with ADHD was significantly higher than those without ADHD
what it looks like is that if you’re untreated with ADHD, then you’re more likely to develop drug dependency, rather than those treated with amphetamine or other drug therapies
Summary
ADHD is characterised by hyperactivity, impulsivity and attentional problems
It affects around ~5% of children/adolescents, and symptoms can continue into adulthood in up to one half of cases
Clinical awareness of the condition dates back to the early years of the 20th century
Term ‘ADHD’ first appeared in the DSM III-R
In general, the condition has a negative effect on:
Academic achievement
Occupational achievement
Social integration
Physical health
Mental health
Classical views (we are referring to them as such, but they aren’t referred to as that in real life circumstances):
classical view 1
it has something to do with the frontal cortex with evidence from neuropsychology, structural imaging, functional imaging
The main neuropsychological evidence is the fact that symptoms of ADHD are similar to the changes following frontal cortex damage
one common consequence of frontal cortex damage is impulsivity, which can be tested in a lab setting by using the IOWA Gambling task
this tests are ability to inhibit gambling actions
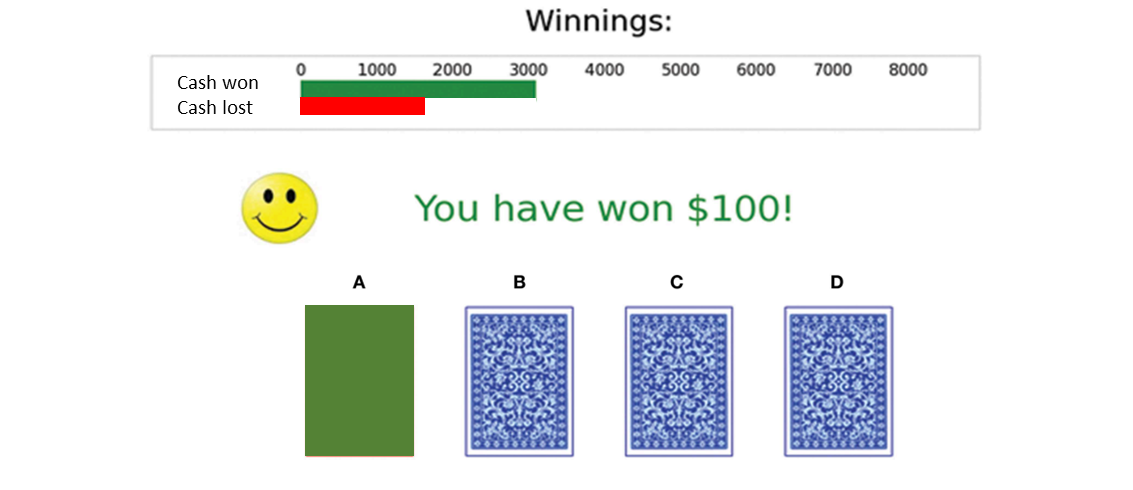
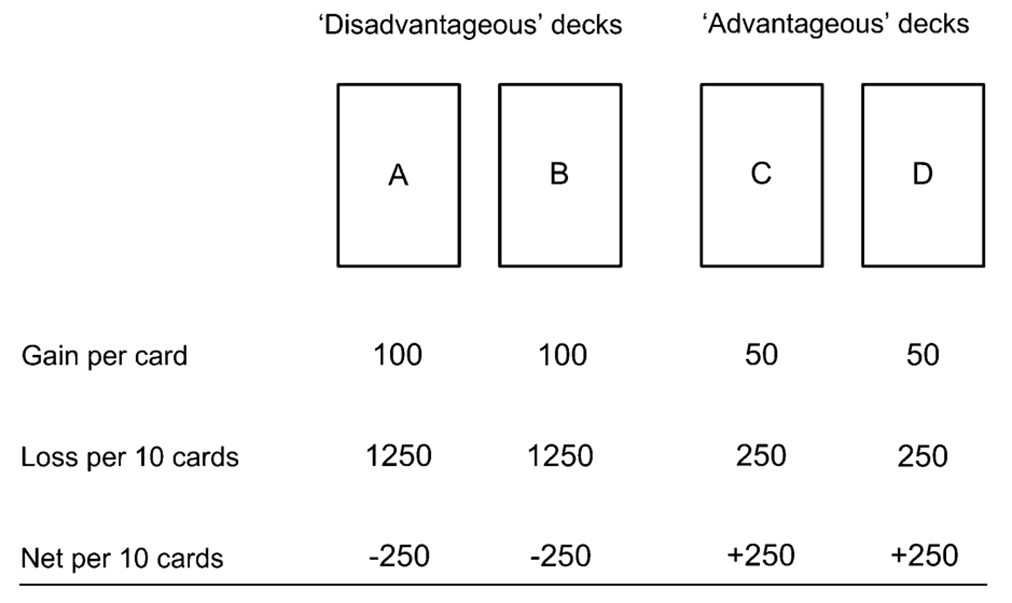
looking at the consequences of turning over: if you turn over the ‘advantageous’ decks you gain £50 but lose £5, whereas if you turn over a disadvantageous deck then you gain £100 but lose £125, tests whether the big gain now is good enough to make people continue to gamble even if their odds and the negative consequences of the other decks are worse
people with frontal cortex damage tended to make more disadvantageous choices than advantageous, they were more seduced by the potential big gain you could make in the moment rather than waiting a little longer and gaining less
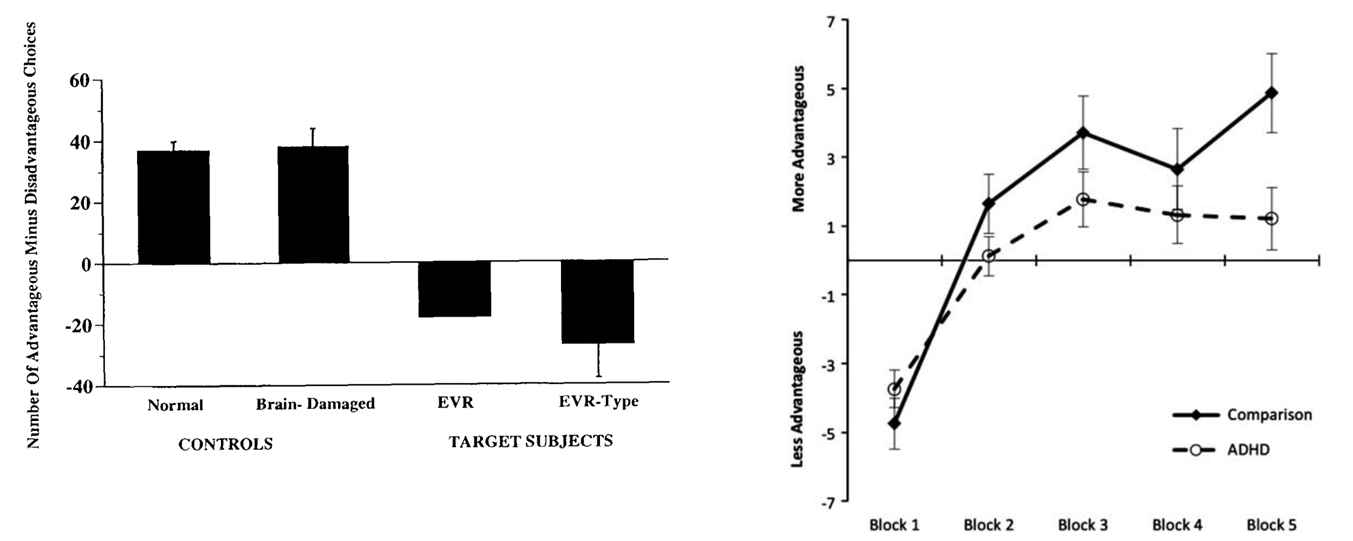
overtime those with ADHD tended to make less advantageous decisions overtime
this distortion of advantageous processing is referred to a ‘delay aversion’ (need the reward now rather than in the future)
Led to Barkley’s ‘behavioural disinhibition’ theory of ADHD (executive)
Russell A. Barkley - Professor of Psychiatry at the Medical University of South Carolina
Sees poor behavioural inhibition as the central deficiency in ADHD
Accounts for attentional and kinetic symptoms
‘Self-control’
Disinhibited behaviour following frontal cortex damage: classic case
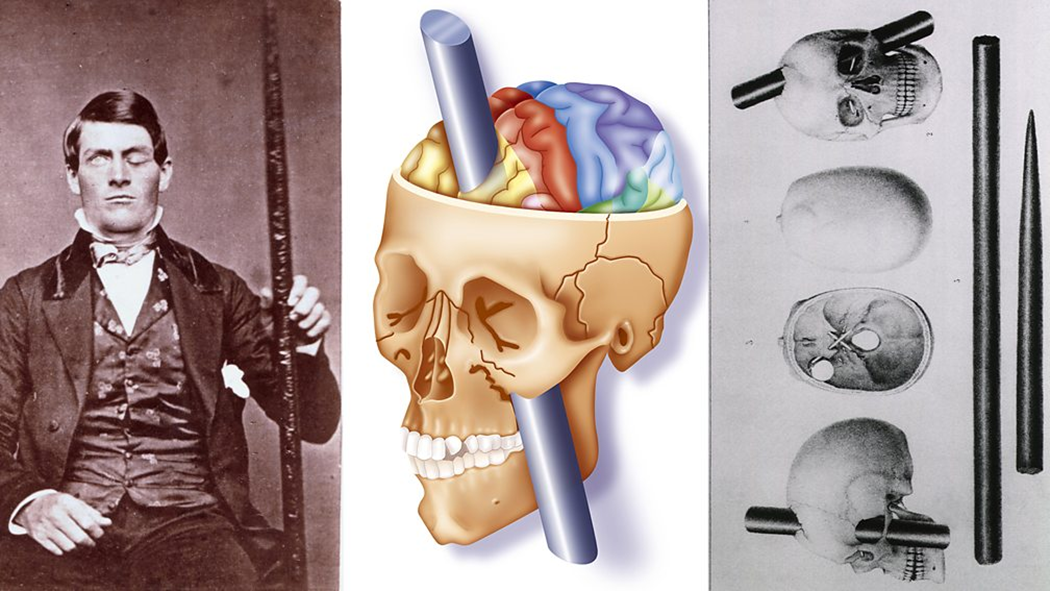
“He is fitful, irreverent, indulging at times in the grossest profanity (which was not previously his custom), manifesting but little deference for his fellows, impatient of restraint or advice….” [Harlow]
Evidence that it has something to do with the frontal cortex: structural (MRI)
frontal lobe grey matter volume is lower…
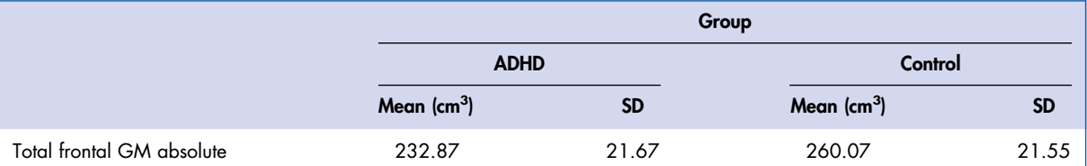
12 kids with ADHD, mean age is 12
mean average of frontal GM amount is much less for those with ADHD
Evidence that it has something to do with frontal cortex damage: functional (PET)
also evidence that the GM left is also less functional, it is hypofunctional
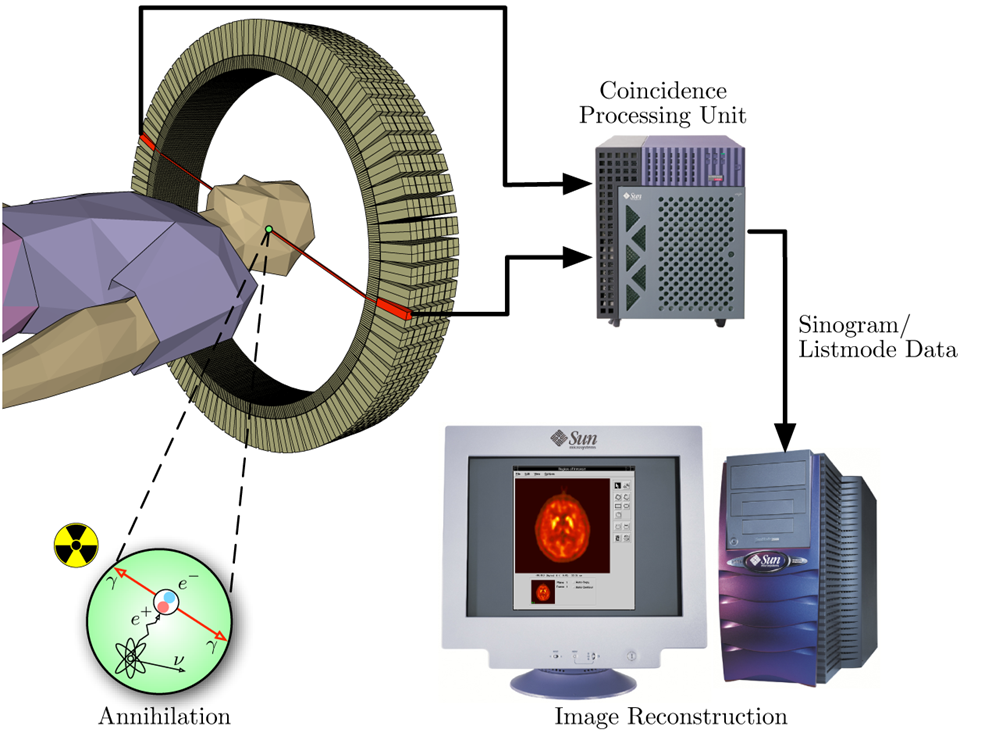
A substance in put in the blood that can be detected in the brain, depending on which part of the brain is active then that part of the brain will receive a significantly larger amount of blood than other areas, so more of the substance too
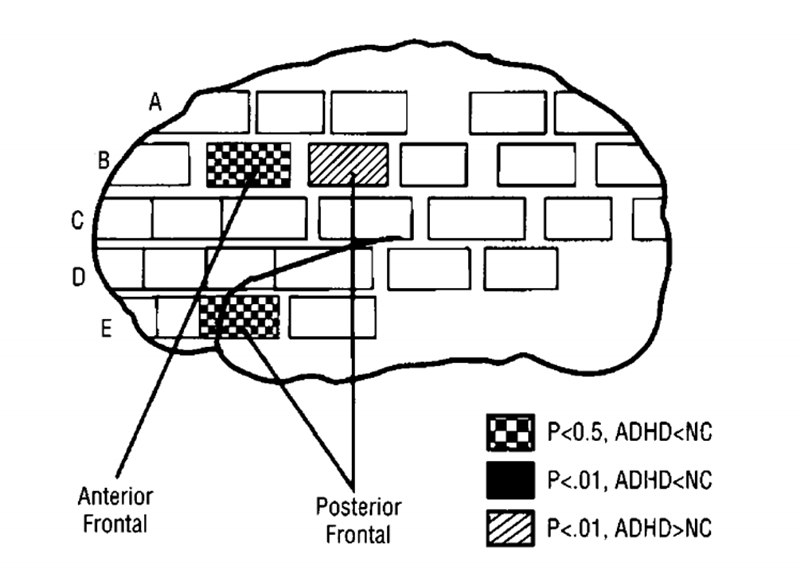
region as of the brain that had significantly different normalised rates of glucose metabolism in patients (teenagers with ADHD) compared with controls
areas of the frontal cortex that were less active in ADHDs than in controls is those areas coloured in a chequered pattern, thus those with ADHD had less active frontal cortex
922,200 people are prescribed methylphenidate per year in the UK
Classical theory 2:
It has something to do with dopamine, as supported by medication, imaging, reward/ reinforcement
evidence 1: Drugs used to treat ADHD increase dopamine levels
microdialysis:
A small, semi-permeable probe is inserted into a specific brain site
Fluid is perfused through the probe and chemicals in the extracellular fluid diffuse across the membrane and are collected
Samples are then analyzed using chromatography methods (e.g. HPLC)
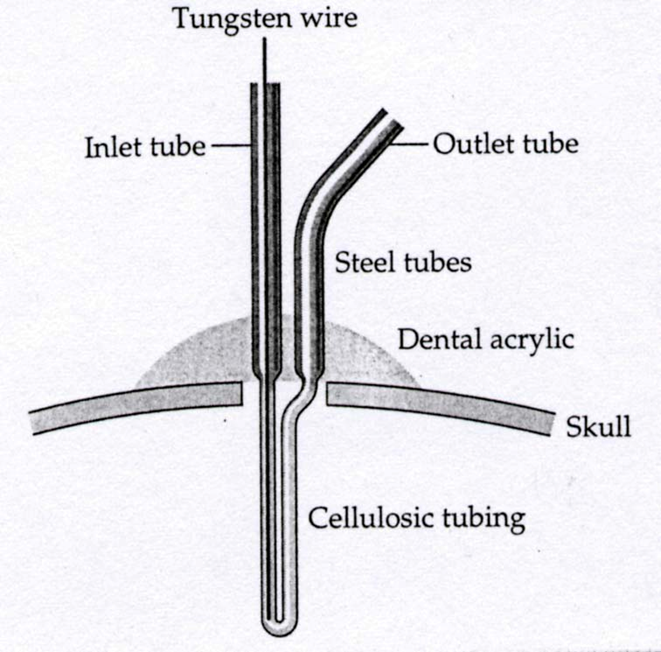
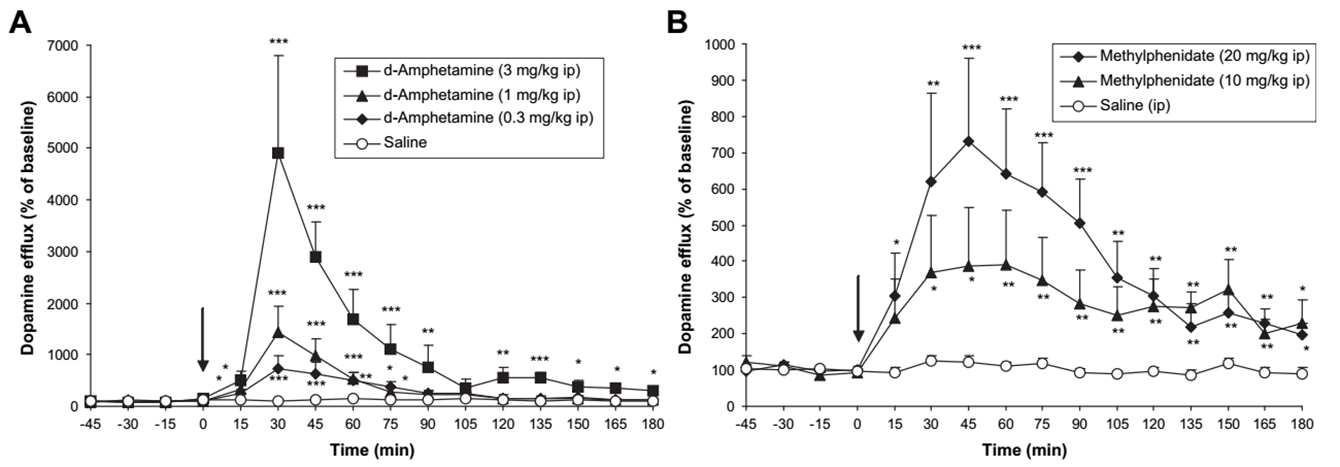
dopamine levels in the striatum after i.. D-amphetamine or methylphenidate
ADHD is associated with an increase in dopamine reuptake
PET
SPECT; a lot cheaper, produces GAMMA rays directly, no need to figure out where within the brain is active, they use a GAMMA camera that pinpoints the area a lot more simplistically
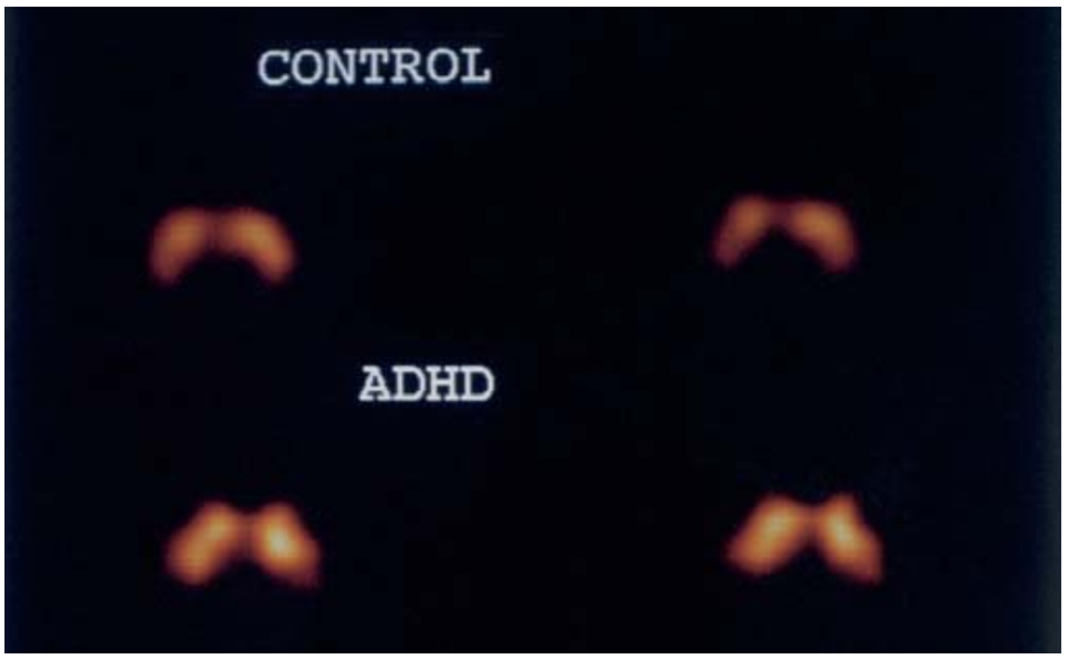
the brighter the image, the more transporters of dopamine the tissue contains, the blobs are the striata which is a large part of the dopaminergic transmission/ neural pathways, because there’s more transporters, the less dopamine you have floating in the brain to be transmitted, the more it is reuptaken by the presynapse potentially, so the levels of dopamine must be lower for those with ADHD than the controls
ADHD is associated with changes in response to ‘reward’
dopamine and the reward of food
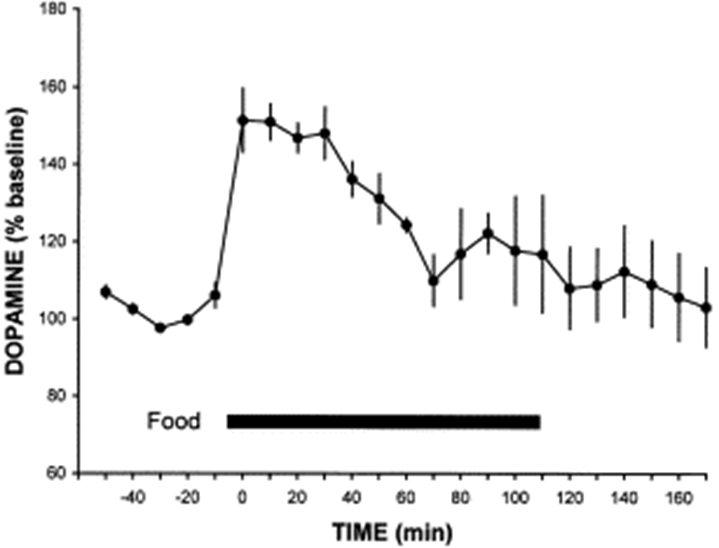
as soon as it was given food, there was a massive increase in the level of dopamine in the animals brain
dopamine and the reward of sex
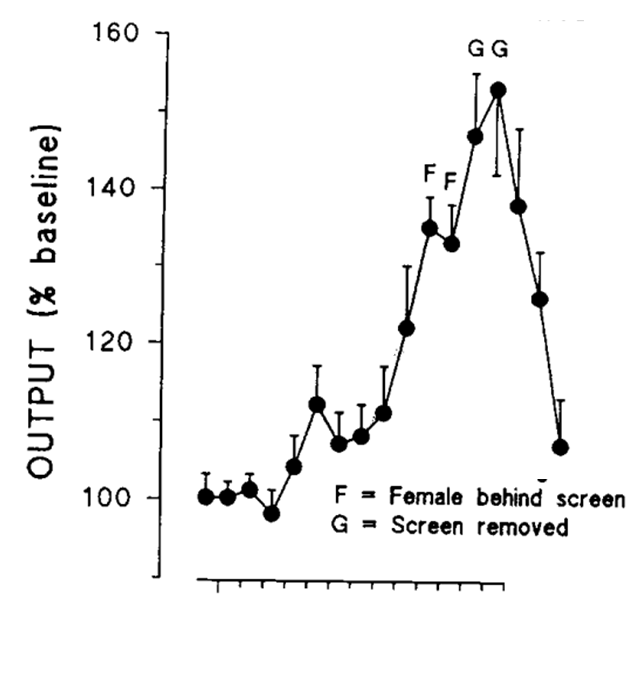
the male and female rats are separated, but there is a massive increase in dopamine levels once the male rat finally sees the female rat
we would expect this to go wrong if this was regarding someone with ADHD
this is what Douglas and Parry found
Douglas and Paryy (1983)
Disruption of learning by non-contingent reinforcement
66 elementary school children (mean age around 9.5 years old)
33 with ADHD, 33 without
Had to release a response key when a stimulus light came on (reaction time task)
3 conditions: rewarded on every trial when they got faster; rewarded on every other trial when they got faster; rewarded randomly (non-contingent)
Reward = “very good”, “excellent” or “very fast”
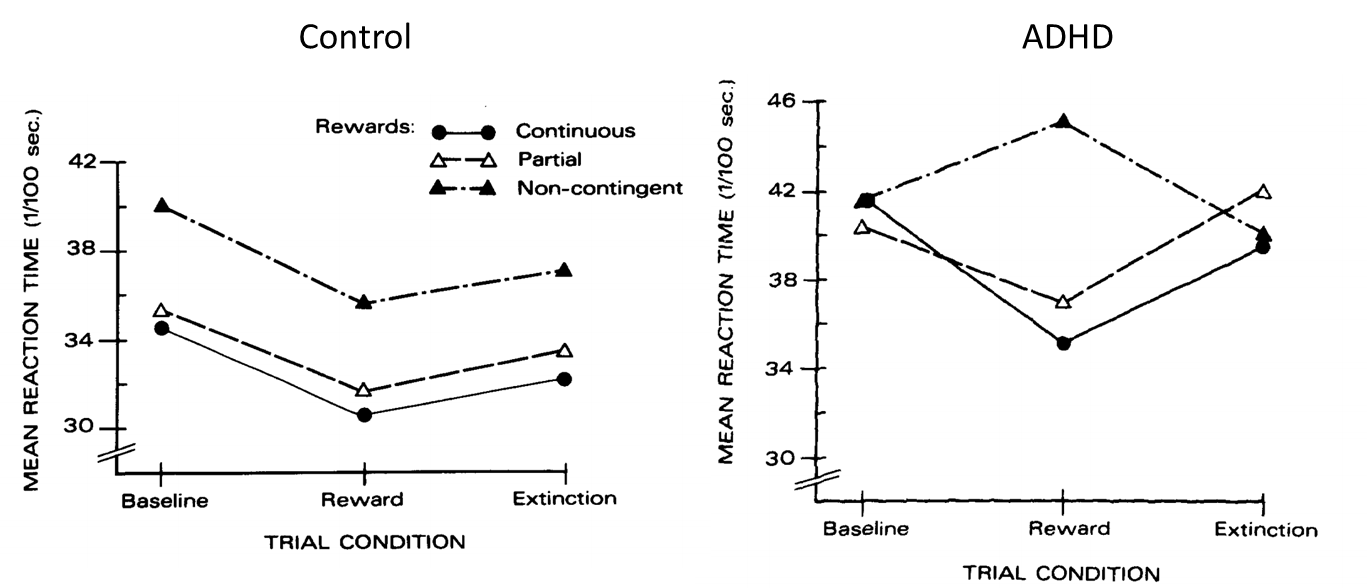
Non-contingent reinforcement schedule resulted in slower mean reaction times in the ADHD sample
(also delay aversion)
Thus classical theories of ADHD suggest:
1. That the disorder has something to do with the frontal cortex
2. That the disorder has something to do with dopamine
In the case of the frontal cortex, evidence comes from:
Neuropsychology; imaging (structural and functional)
In the case of dopamine, evidence comes from:
Medication; imaging (SPECT); reward/reinforcement
Reading
Thapar A, & Cooper M. (2016) Attention deficit hyperactivity disorder. Lancet, 387(10024):1240-50.
introduction
Attention deficit hyperactivity disorder (ADHD) is a childhood-onset neurodevelopmental disorder characterised by developmentally inappropriate and impairing inattention, motor hyperactivity, and impulsivity, with difficulties often continuing into adulthood.
definitions of ADHD
DSM-4 Vs DSM-5 Vs ICD-10
ADHD is a diagnostic category in the DSM-IV and DSM-5. The broadly equivalent is hyperkinetic disorder as defined in WHO’s International Classification of Diseases (Europe). This definition includes a more severely affected group of individuals, as prevalence of hyperkinetic disorder is lower than DSM-IV ADHD. DSM-5 has longer symptom descriptors than those in DSM-IV. DSM-IV distinguished between inattentive, hyperactive–impulsive, and combined subtypes of ADHD; a diagnosis of the combined subtype required symptoms across inattention AND hyperactivity–impulsivity. However, ADHD subtypes are not stable across time, and DSM-5 de-emphasised their distinctions. ICD-10 does not distinguish subtypes; symptoms need to be present from the three separate domains of inattention, hyperactivity, and impulsivity for hyperkinetic disorder. The diagnosis of ADHD or hyperkinetic disorder also requires the presence of symptoms across more than one setting and requires symptoms to result in impairment, like in academic, social, or occupational functioning. Onset must be early, although DSM-5 has changed the age of onset from before 7 (ICD-10 and DSM-IV) to before age 12. Individuals with ADHD differ in core symptom combinations, level of impairment and comorbidities, as well as on background individual, family, and social factors. For clinical purposes, defining ADHD categorically is useful as clinical decisions tend to be categorical in nature—eg, whether to refer to specialist services or to treat. However, like many medical disorders, in terms of causes and outcomes, ADHD can be viewed as a continuously distributed risk dimension. Perhaps there is a lack of an objective cut-point that defines the diagnostic threshold. Individuals with subthreshold symptoms are at risk of adverse outcomes. However, categorical decisions on resource allocation and treatment have to be made, and ICD-defined or DSM-defined diagnosis provides a reliable way of balancing the risks and benefits of giving an individual a diagnostic label and providing treatments that are not free of adverse effects. A further challenge comes from the diagnosis being based on reported symptoms alone with no biological tests. This means that even with clear-cut diagnostic criteria, there is risk of overdiagnosis and underdiagnosis, which underscores the importance of rigorous expert assessment.
Epidemiology
The general estimated prevalence of ADHD in children is 3·4% with lower rates of around 1·4% reported for hyperkinetic disorder from European studies. Prevalence does not vary by geographical location but is affected by heterogeneity in assessment methods (eg, use of an additional informant to the parent or carer) and diagnostic conventions (eg, ICD vs DSM). Notably, there is a marked under-representation of studies on ADHD from low-income and middle-income countries.
One common assumption is that ADHD must be a modern occurrence. Time trends studies of in the late 20th and early 21st centuries show no evidence of a rise in rates of ADHD symptoms or diagnosis across past decade. However, there has been a very marked rise in the number of prescriptions issued for ADHD pharmacological treatment across high-income countries in the Rises in clinic incidence and treatment could simply indicate increased parent and teacher awareness of ADHD or changes in the impact of symptoms on children’s functioning, or both. Nevertheless, despite the rise in ADHD treatment, the administrative prevalence is lower than the population figure, highlighting that in these countries there is still underdiagnosis. However, in the USA, studies show geographical variation in patterns of underdiagnosis and overdiagnosis or in ADHD medication prescribing. Highlight that there is the potential for misdiagnosis and inappropriate use of pharmacological interventions if safeguards are not in place. These safeguards include ensuring full, good-quality clinical assessments are undertaken, even though these require time, and adherence to national and international treatment guidelines. However, there is no evidence of rising population rates of ADHD explained by social change.
Although the male: female ratio of 3–4:1 recorded in epidemiological samples is increased in clinic populations to around 7–8:, suggesting referral bias to female patients with ADHD. The same is seen for other neurodevelopmental disorders such as autism spectrum disorder, intellectual disability, and communication disorders.
The natural history of ADHD is best examined in prospective longitudinal studies. As is typical of neurodevelopmental disorders, the core defining features of ADHD tend to decline with age, although inattentive features are more likely to persist. However, the developmental trajectories of ADHD are highly variable. Although around 65% of patients continue to meet full criteria or have achieved only partial remission by adulthood, some patients do achieve full remission. Good-quality, large epidemiological studies of the prevalence of ADHD in adulthood are lacking, but one meta-analysis of adult ADHD yielded a pooled prevalence of 2·5%. DSM-5 explicitly allows for symptom decline and requires a reduced number of symptoms for diagnosis of adult ADHD. In clinical settings, diagnosis of ADHD in adults who did not present in childhood requires some caution in the absence of documented information because of the difficulty for young adults and those who know them to date symptom onset retrospectively. Objective records (eg, school reports) could help. There is sufficient evidence that ADHD is not simply a problem that most children grow out of. However, transitioning from child to adult mental health clinics is difficult because of a scarcity of adult services.
Key diagnostic symptoms of attention deficit hyperactivity disorder
inattentive symptoms
Does not give close attention to details or makes careless mistakes
Has difficulty sustaining attention on tasks or play activities
Does not seem to listen when directly spoken to
Does not follow through on instructions and does not finish schoolwork, chores, or duties in the workplace
Has trouble organising tasks or activities
Avoids, dislikes, or is reluctant to do tasks that need sustained mental eff ort
Loses things needed for tasks or activities
Easily distracted
Forgetful in daily activities
hyperactivity or impulsivity symptoms
Fidgets with or taps hands or feet, or squirms in seat
Leaves seat in situations when staying seated is expected
Runs about or climbs when not appropriate (may present as feelings of restlessness in adolescents or adults)
Unable to play or undertake leisure activities quietly
“On the go”, acting as if “driven by a motor”
Talks excessively
Blurts out answers before a question has been finished
Has difficulty waiting his or her turn
Interrupts or intrudes on others
Early comorbidity
ADHD shows high concurrent comorbidity with other neurodevelopmental disorders— autism spectrum disorder, communication and specific learning or motor disorders, intellectual disability, and tic disorders. Rates of comorbidity are higher in individuals clinically referred than those who are not referred. ADHD also shows high concurrent comorbidity with behavioural problems— namely, oppositional defiant and conduct disorders. Conduct disorder is a risk marker for greater neurocognitive impairment and worse prognosis in children with ADHD. This subgroup of children with hyperkinetic conduct disorder is distinguished in ICD-10 but not in DSM-5.
Risk factors: genetics
ADHD is a familial disorder. Its relative risk is about 5–9 in first-degree relatives of probands with ADHD. Many twin studies of ADHD have yielded high heritability estimates of about 76%, a magnitude similar to schizophrenia and autism. The genetic architecture of ADHD is similar to other neuropsychiatric disorders such as schizophrenia. Several different classes of genomic variants have been identified to be associated with ADHD risk. These variants include common DNA sequence variants called single nucleotide polymorphisms (SNPs), but associations have only been reported when thousands of SNPs are combined into a composite genetic risk score. Subtle chromosomal mutations, such as rare deletions and duplications called copy number variants (CNVs), are also associated with ADHD risk. These have larger effect sizes but are uncommon. Before whole-genome investigations, specific single dopaminergic, serotonergic, and noradrenergic candidate genes were significantly associated with ADHD status in meta-analyses. However, in the present era of whole-genome investigation, psychiatric candidate gene studies of DNA variants in single genes are viewed with caution because of the potential for false positives. ADHD-associated genomic variants are non-specific; composite genetic risk scores show significant overlap with those contributing to schizophrenia and mood disorders. ADHD-associated CNVs also show overlap with ones associated with schizophrenia, autism, and intellectual disability. Although testing for rare CNVs is now recommended for individuals with intellectual disability, this is not the case for ADHD. Although most cases of ADHD are multifactorial in origin, there are several known, rare genetic syndromes (such as fragile X syndrome, tuberous sclerosis, 22q11 microdeletion, and Williams syndrome) characterised by high rates of ADHD and ADHD-like features. These syndromes are associated with high risk of other disorders, such as autism (especially in fragile X syndrome and tuberous sclerosis) and schizophrenia (22q11 microdeletion syndrome). In typical clinic populations with ADHD, there is no evidence that routine screening for these genetic syndromes is warranted in the absence of intellectual disability.
environment and G-E interplay
Observational case-control and epidemiological studies show that exposures to a range of prenatal and perinatal factors, environmental toxins, dietary factors, and psycho social factors are all associated with ADHD. If these associations are causal, it means manipulation of the risk factor can alter the outcome. However, association does not mean causation, because exposures to risks are not randomly allocated and can be affected by unmeasured confounders, selection factors, and reverse causation, whereby the phenotype influences the environmental exposure.
Prenatal and perinatal factors reported to be associated with ADHD are low birthweight and prematurity, and in-utero exposure to maternal stress, cigarette smoking, alcohol, prescribed drugs (eg, paracetamol), and illicit substances. In relation to prenatal smoking and stress, most/all of the association with off spring ADHD, unlike off spring birthweight, is explained by unmeasured confounding factors. Environmental toxins, specifically in-utero or early childhood exposure to lead, organophosphate pesticides, and polychlorinated biphenyls, are risk factors for ADHD. Nutritional deficiencies (eg, zinc, magnesium, and polyunsaturated fatty acids), nutritional surpluses (eg, sugar and artificial food colourings), and low or high IgG food have not been shown convincingly to precede ADHD. Psychosocial risks, such as low income, family adversity, and harsh or hostile parenting, although robustly causal for some psychiatric disorders, are also correlates rather than proven causes of ADHD.
Longitudinal studies, treatment trials, and a study of children adopted away at birth suggest that observed negative mother–child relationships (even in unrelated mothers) arise as a consequence of early child ADHD symptoms (reverse causation) and improve with treatment. However, exposure to very severe, early social deprivation seems to be diff erent and causal. After being adopted away in the UK, Romanian orphans raised in institutions and exposed to extreme early privation in the first year of life showed increased rates of ADHD-like features, cognitive difficulties, and quasi-autistic features that persisted into adolescence. Psychosocial context may well shape ADHD presentations and alter developmental trajectories, outcomes, and impairments, but this has not been investigated.
Treatment is based on clinical features and not assumed aetiology. Potentially important environmental risks for ADHD and its outcomes may be brought about as a consequence of genetic propensities (gene–environmental correlation). The effect of environmental factors on clinical phenotype may also depend on genetic liability. For example, animal studies have robustly shown that environment can alter behaviour in different ways depending on the variant of gene carried (gene–environment interaction). Effects of gene–environment interplay are subsumed in twin heritability estimates. Finally, there is very good evidence that environmental exposures result in biological including ones involving brain structure, changes, function, and altered DNA methylation (epigenetics).
Pathophysiology- biology
The biological mechanisms are not yet understood and there remains no diagnostic neurobiological marker. The validity of animal models of ADHD are limited by our incomplete understanding of its pathophysiology in man and the extent to how well inattention, motor overactivity, and impulsive responses on behavioural tasks in non-human species represent ADHD. However, findings in animal models have suggested involvement of dopaminergic and noradrenergic neurotransmission (in line with the neurochemical effects of ADHD medications) as well as involvement of serotonergic neurotransmission.
pathophysiology- cognition
Although there is no cognitive profile that defines ADHD, deficits in various neuropsychological domains have been reliably identified. In terms of executive functioning, the most consistent and strong associations are seen for response inhibition, vigilance, working memory, and planning. In terms of non-executive deficits, associations are seen with timing, storage aspects of memory, reaction time variability, and decision making. However, there is substantial heterogeneity in cognitive functioning even within single samples, and there is not a straightforward association between cognitive performance and the trajectory of clinical symptoms. There is evidence, though, that some cognitive deficits are improved by methylphenidate, with a meta-analysis showing improvements in executive and non-executive memory, reaction time, reaction time variability, and response inhibition.
Imaging
Functional MRI (fMRI) studies have found abnormalities in the function of many neural networks in response to cognitive tasks. A meta-analysis identified alterations in several networks, including those related to attention and executive function. In terms of brain structure, a meta-analysis of structural MRI studies highlighted alterations in the basal ganglia and limbic areas. In a meta-analysis of diffusion MRI studies investigating white matter microstructure, alterations were reported to be widespread, but most reliably seen in the right anterior corona radiata, right forceps minor, bilateral internal capsule, and left cerebellum. Reduced total grey matter and altered basal ganglia volumes seem to index familial risk for ADHD. This is increasingly suggesting that the pathophysiology of ADHD involves abnormal interactions between large-scale brain networks; however, current imaging studies do not yet have relevance to clinical practice. Interpretation is complex because of the cross-sectional nature of most studies. Longitudinal data regarding the trajectory of cortical development suggest that the brain may show maturational delay, with persistence of ADHD indexed by progressive divergence from the normal trajectory, but it is not known whether this phenomenon can be extrapolated to other metrics of structure, microstructure, and function. The effect of pharmacological treatment is also a consideration as some evidence suggests that it normalises macrostructure and function. Nonetheless, there is some evidence from longitudinal studies of adults with childhood ADHD that grey and white matter abnormalities persist well into adulthood.
clinical assessment
The assessment process requires goes beyond yes or no questions in relation to core ADHD symptoms. A missed diagnosis can jeopardise an individual’s learning or occupational and social relationships, whereas a misdiagnosis may lead to pharmacological treatment that is not needed. History taking should not be reductionist—ie, exclusively focused on asking about diagnostic items. A detailed developmental as well as medical history and an assessment of family processes and social circumstances are also required. It is important whether endorsed symptoms are better explained by difficulties amenable to intervention—eg, hearing difficulties presenting as inattention. However, diagnosis is based on clinical phenotype and not generally excluded by presumed cause. Information should be obtained from multiple informants. To decide which individuals need referral to a specialist assessment service or to monitor treatment response, use of standardised ADHD questionnaires are helpful, but are not a substitute for detailed history taking before diagnosis. Structured interviews are more likely in a research setting, but may be valuable in a clinical context, especially interviews that do not require extensive, expensive training (eg, the Development and Wellbeing The use of structured interviews in a Assessment). ADHD symptoms are commonly associated with neurobehavioural difficulties, which could be comorbid features but should be considered as differential diagnoses because treatment is different. Mental health symptoms, which should be screened for, include oppositional defiant disorder, conduct disorder, anxiety, and mood disturbance. Developmental and learning problems such as reading disorders, developmental coordination disorder, and tic disorders are also common. Because ADHD and autism spectrum disorder co-occur autistic symptomatology should be considered. ADHD is associated with intellectual disability and emotion dysregulation symptoms (eg, irritability), which can complicate the presentation and interpretation of symptoms. In practice, it is rare to find an individual with so-called uncomplicated ADHD. This situation makes formalising differential diagnoses conceptually difficult, because in reality an individual with neurodevelopmental problems is unlikely to have a pure presentation of one condition as an explanation for their difficulties. A formulation should capture the full range of developmental, behavioural, and psychiatric difficulties, even if some need to be described in terms of subthreshold problems. Neuropsychological testing does not have a role in diagnosis of ADHD because cognitive processes are not a defining characteristic. However, cognitive comorbidities such as learning disability and dyslexia should be considered.
summary of the clinical assessment process for children
obtain detailed clinical history from parents or carers and young person → Carry out core ADHD symptom enquiry: are symptoms out of keeping with child's age and developmental stage? → Obtain information across settings; consider questionnaires as an adjunct → Screen for associated difficulties (eg, mental health symptoms, other neurodevelopmental or learning problems) → Developmental history (eg, motor delay), Medical history (eg, epilepsy), Family history (eg, mental health, educational history, physical health problems), Medical histories especially important in relation to cardiac or other risk factors if pharmacological treatment is being considered → Consider severity of symptoms, effects on functioning, comorbid symptoms, medical history, and the family and child's strengths, resources, demands, and psychosocial context when deciding on treatment options → Physical assessment: • Signs of other disorders (eg, dysmorphic features, skin lesions) and motor coordination (eg, handwriting, balance); to be undertaken more completely if considering pharmacological treatment • Baseline height, weight, blood pressure, pulse
Treatment
There are specific guidelines for the management of ADHD, such as those developed by NICE and SIGN in the UK, by the Eunethydis European ADHD Guidelines Group (EAGG) in Europe, and by the American Academy of Pediatrics (AAP) and the American Academy of Child and Adolescent Psychiatry (AACAP) in the USA. The main difference is that US guidance does not preclude pharmacological treatment for preschool children or those with mild ADHD; practice that is not recommended in Europe where a stepwise approach is recommended. If pharmacological treatment is prescribed, it should be with behavioural interventions—like, optimised classroom management strategies, parental psycho education, and behavioural management techniques.
Individual circumstances such as current academic or employment demands and medical history should be taken into account, and appropriate evidence-based treatments for comorbidities should also be initiated. The only nonpharmacological interventions that currently form a core part of treatment guidelines are behavioural interventions. Initial results from the multimodal treatment study of children with ADHD (MTA), suggested that the combination of intensive behavioural treatment and pharmacological treatment did not offer benefit over pharmacological treatment alone for core ADHD symptoms, but that the combination might provided some benefit in terms of associated symptoms, levels of functioning and the need for a lower drug dose. In meta-analyses it was concluded that, along with neurofeedback, cognitive training, and restricted elimination diets, behavioural interventions cannot be recommended as interventions for core ADHD symptoms until better evidence of their effectiveness is reported by blinded assessments. No artificial food colouring may be beneficial, but to what extent and for whom is unclear. A meta-analysis showed that children with ADHD have lower concentrations of omega-3 fatty acids than controls and that supple mentation improves ADHD symptoms to a modest degree. However, there is evidence of a beneficial effect of behavioural interventions on parenting and child conduct problems, and that CBT may be useful for adults with ADHD when used with pharmacological treatment. Stimulants like methylphenidate and dexamfetamine are the first-line pharmacological treatments for ADHD, and the noradrenaline reuptake inhibitor atomoxetine is the second-line treatment.
Each of these treatments increases catecholamine availability. Meta-analyses have provided evidence for the efficacy of stimulants for ADHD in children, in children with co-occurring ASD, and in adults. Although it is recommended that ADHD is treated in individuals with autism spectrum disorder or intellectual disability, or both, side-effects of pharmacological treatment in these individuals are more common than in those with ADHD alone. Meta-analyses have shown beneficial effects of atomoxetine in children and in adults. Extended release guanfacine and extended-release clonidine are licensed in the USA. Pretreatment checks, including in relation to medical and family medical history (in particular cardiac disorders), are important if medication is taken. Height, weight, blood pressure, and pulse should be checked at baseline before starting treatment, and compared with normative data. It is reasonable to consider the routine performance of an ECG before starting pharmacological treatment, and should be at the treating clinician’s discretion, taking into account factors such as medical history, family medical history, and physical examination findings. It is best to start with a low dose, titrate up according to response, and monitor side-effects. There is no evidence that pharmacological treatment for ADHD is associated with changes in QT interval, sudden cardiac death, acute myocardial infarction, or stroke.
Once an optimum response is achieved, height, weight, and growth will need regular monitoring. NICE guidance recommends that height is measured every 6 months in children and young people; weight is measured 3 months and 6 months after initiation of treatment and every 6 months thereafter in children, young people, and adults; and height and weight in children and young people should be plotted on a centile chart. Blood pressure and pulse should also be plotted before and after each change in dose and routinely every 3 months. Adverse side-effects of stimulant medication for ADHD include appetite suppression and growth retardation, which can be off set by stimulant holidays on days when symptom control is deemed less crucial, such as weekends and holidays, and by adjusting the timing of doses. Other side-effects of stimulants and atomoxetine include gastrointestinal symptoms, cardiac problems, insomnia, and tics (but tics are less common with atomoxetine). Stimulants are controlled drugs with potential for diversion for misuse, and if there is a concern then an alternative drug may be preferable.
prognosis
Not only do core ADHD symptoms themselves persist, but individuals with childhood ADHD are also at substantial risk of adverse outcomes in adolescence and adulthood. ADHD behaves dimensionally: there is no distinct threshold where adverse outcomes appear. A diagnosis of ADHD is associated with low academic attainment and premature cessation of education, and poor educational outcomes also extend to individuals with subthreshold symptoms.
ADHD predicts serious antisocial behaviour, involvement with the police, and substance misuse in adolescence. Until recently, few data for broad outcomes beyond the third decade of life were available. However, one long-term follow-up study has shown that childhood ADHD (participants aged 6–12 years) is also associated with adverse occupational, economic, and social outcomes, antisocial personality disorder, and risk of substance use disorders, psychiatric hospital admissions, incarcerations, and mortality. A Danish registry-based investigation showed substantially increased mortality in adult life in individuals with ADHD. This increase in mortality was mainly a result of accidents, and especially increased in those with comorbid oppositional defiant disorder, conduct disorder, and substance misuse. A meta-analysis of ADHD in prison inmates showed an estimated prevalence of 30·1% in youth prison populations and 26·2% in adult populations, with the risk for female prisoners being nearly as high as that for male prisoners. Psychiatric comorbidity is high in prisoners with ADHD, especially in adults. There is epidemiological evidence that pharmacological treatment might reduce criminal behaviour and trauma-related visits to emergency departments.
Most people with ADHD do not develop psychosis or a mood disorder. Studies only find a small subgroup of individuals who additionally develop schizophrenia or bipolar disorder. Evidence between ADHD and later unipolar depression is inconsistent; may be because depression is more common in female patients who are under-represented in ADHD samples.
Future research and clinical directions
The early age of onset, male preponderance, and strong comorbidity with other childhood-onset neuro developmental disorders support the inclusion of ADHD in the DSM-5 grouping of neurodevelopmental disorders. The previous practice of not diagnosing ADHD in the presence of autism spectrum disorder or intellectual disability has been a crucial barrier to research on aetiological and clinical overlaps and distinctions as well as to clinical and educational practice. Unfortunately, referral and treatment pathways and service provision in health and education tend to be diagnostically focused (ie, autism only or intellectual disability only), although some clinics and services are focusing more broadly on childhood neurodevelop mental disorders, a change which is welcomed and supported by research. We accept that for clinical practice, there is a need for strict categories, otherwise diagnostic spread would become at best unhelpful and at worst risky and unethical (eg, use of pharmacological treatment where not indicated) and application of evidence-based treatments would become impossible (eg, interpreting the severity of difficulties of individuals included in a trial). However, for aetiological and outcome research purposes, there is strong evidence in favour of viewing ADHD dimensionally. At present, we do not know what sorts of dimensions best capture ADHD and at what level they should be measured—eg, reported symptoms, cognitive tests, brain imaging markers, or other biological signatures. Genetic research is progressing via large-scale collaboration, but there is a need to understand the clinical as well as biological meaning of findings, if they are to affect our understanding and treatment of ADHD. Currently, there is no rationale for routine genetic testing in ADHD because of limited predictive power. However, because the disorder is heritable, rates of ADHD in parents of those with ADHD are increased. A pertinent future research question is how might treatment of parent ADHD affect child ADHD features and comorbidity? There is, for example, evidence that treating parent depression seems to improve off spring mental health. Another issue for future consideration is that genetic and environmental risk factors that cause ADHD are not necessarily the same as those that alter the later course of the disorder or contribute to adverse outcomes. What is greatly needed is research that tests which environmental risks (eg, social and other potentially modifiable risk factors) contribute to and modify the longitudinal course of ADHD across time, including better prognosis, with designs that can control for unmeasured confounders and genetic contributions from the affected person (eg, twin studies) and related parents (eg, adoption studies). This could inform interventions aimed at optimising outcomes. So far, pharmacological and behavioural treatments for ADHD have focused on symptomatic relief of the core symptoms of inattention, overactivity, and impulsivity. However, according to trial-based data, benefits seem to be short-lived. Another issue is that treatment typically begins after a child has already begun to fail across multiple domains. ADHD in many respects behaves like a chronic medical disorder. Many features remain problematic long term, although the most prominent or presenting features can change with age and development. ADHD creates risks of its own and secondary mental health problems commonly arise in mid-childhood and after puberty. Almost certainly, for many individuals, multimodal interventions that are carefully adjusted over time to prevent complications will be needed, perhaps in the way that is undertaken for optimising diabetes control. How ADHD is best managed across the lifespan and across key transition periods (eg, school entry, comprehensive or high-school entry, transition to adult services, and transition to parenthood) needs much more investigation. Until now, guidelines have been based on evidence, but unless research keeps pace, guidance will have to be based on professional consensus, which is not very satisfactory for a prevalent, impairing disorder.
conclusions
ADHD is a very important condition because of its high prevalence, persistence into adult life, and adverse outcomes that extend beyond the affected individual. Although ADHD is still viewed with scepticism by some and often remains stigmatised by the media, the evidence for it being a clinically and biologically meaningful entity is robust and consistent across design type and sample. There are established assessment methods and good treatment evidence. However, as is true for any chronic disorder, repeated assessment is likely to be needed and treatment will typically need many adjustments over time. Impairments beyond core diagnostic criteria, developmental change, and an individual’s psychosocial strengths, weaknesses, and resources are all important aspects for consideration