Campbell Unit 1: The Chemistry of Life
Chapter 1: Evolution, the Themes of Biology, and Scientific Inquiry
1.1: The study of life reveals unifying themes
Biology, the study of life, has enormous scope with five unifying themes: organization, information, energy/matter, interactions, and evolution.
Organization: Hierarchy of biological organization
1. Biosphere: All life on earth and all the places where life exists
2. Ecosystems: Consists of all living things in a particular area, along with nonliving parts that life interacts with (ex. North American mountain meadow)
3. Communities: Array of organisms inhabiting an ecosystem (ex. plants, animals, fungi, bacteria, etc. in meadow)
4. Populations: All individuals of a species living within same area that interbreed with each other, set of populations that inhabit an area is community (ex. lupine flower in meadow)
Species: Can only reproduce with each other
5. Organisms: Individual living things (ex. each plant in meadow)
6. Organs: Body part made of multiple tissues with specific functions in the body (ex. lupine leaf)
7. Tissues: Group of cells that work together to perform a specific function (ex. honeycombed tissue of lupine leaf)
8. Cells: Life’s fundamental unit of structure and function
9. Organelles: Various functional components present in cells (ex. chloroplasts)
10. Molecules: Chemical structure consisting of 2+ atoms (ex. the atoms that make up chlorophyll)
Emergent Properties: Properties emerge at each level that weren’t there on the last one, due to arrangement and interactions of parts as complexity increases
Systems Biology: Analyze interactions among parts of system
Cell Theory: All living organisms made of cells, and actions are based on cell activity
Eukaryotic Cell: Membrane enclosed organelles and nucleus
Prokaryotic Cell: No nucleus or membrane enclosed organelles, smaller than eukaryotic
Chromosomes contain genetic material as DNA (deoxyribonucleic acid), which has hundreds or thousands of genes
Gene Expression: The process which genetic information directs the production of a cellular product
Genome: “Library” of genetic instructions that were inherited
Genomics: Studying sets of genes in one or more species at once
Proteomics: Studying of sets of proteins at once
Proteome: Entire set proteins
Three research developments made these two approaches possible:
“High throughput” technology which analyzes many samples quickly
Bioinformatics: Use of computational tools to store, organize, and analyze data from this technology
Formation of interdisciplinary teams (groups of diverse specialists)
Chemical energy goes from sun > producers > consumers
Energy flows in one direction
Chemicals go in a cycle, eventually returned to environment by decomposers
At lower levels of organization, interactions are crucial for smooth operation
Feedback Regulation: Output of product regulates it
Negative Feedback: Most common, Response reduced initial stimulus
Positive Feedback: Product speeds up own production
A molecule consists of atoms bonded together
a. Life’s Processes Involve Expression and Transmission of Genetic Information ,b. New Properties Emerge at Successive Levels of Biological Organization, c. Life Requires the Transfer and Transformation of Energy and Matter
1.2: The core theme: Evolution accounts for the unity and diversity of life
Evolution: As species’ adapt to different environments they become more and more different from their ancestors over time
Three domains:
Bacteria & Archaea: Prokaryotic & Single Cell
Eukarya: Eukaryotes
Includes kingdom Plantae, kingdom Fungi, kingdom Animalia, & Protists
Distinguished (partly) by modes of nutrition
Plants produce use photosynthesis
Fungi absorb dissolved nutrients from surroundings
Animals eat and digest other organisms
Protists: Mostly single celled, most numerous & diverse group
Natural Selection: Certain traits give organisms advantages over others
Nature selects traits which become advantageous, essentially letting it to select which traits to incorporate into the population.
The bird would be able to reach seeds that are further in and harder to reach
1.3: In studying nature, scientists form and test hypotheses
Science: Our approach to understand the natural world
Inquiry: Search for information and explanations of nature
Data: Recorded observations
Inductive Reasoning: Collecting and analyzing observations
Hypothesis: Explanation based on observations and assumptions
Experiment: Scientific test carried out under controlled conditions
Deductive Reasoning: General > Specific
Opposite of inductive
If…then
Theory: Broader scope than hypothesis, general enough to have sub-hypotheses, more evidence than hypothesis
Color is camouflaged > % camouflaged numbers
Inductive is specific to broad, deductive is broad to specific
In sandy areas, they’ll be a sandy color, in black rock areas, they’ll be dark. This is because of natural selection—the better camouflaged, the better chances to survive to reproduce and pass on the genes with the fur color alleles.
1.4: Science benefits from a cooperative approach and diverse viewpoints
Model Organism: Species easy to grow in a lab and easy to investigate
Chapter 2: The Chemical Context of Life
2.1: Matter consists of chemical elements in pure form and in combinations (compounds)
Matter: Takes up space and has mass
Element: Substance that can’t be broken down into other elements
Compound: A substance with 2+ elements combined in a fixed ratio
20-25% of the 92 elements are essential elements needed for an organism to survive and reproduce (varies between species)
Trace Elements: Required by organism only in small quantities
When the two elements are combined to form a compound, it becomes edible. The edibility of table salt is an emergent property, since neither sodium or chlorine was previously edible.
A trace element is an essential element, since even thought it is only required in small quantities, it is still required to survive.
An iron deficiency might lead to decreased oxygen flow and prevent cellular respiration, as well as the production of ATP because of it
After a mutation, over time, the organism with the mutated gene might be able to survive and reproduce, eventually spreading its genes to the entire rest of the population.
2.2: An element’s properties depend on the structure of its atoms
Atom: Smallest unit of matter that still has the properties of element
Composed of subatomic particles, including protons (+), neutrons, and electrons (-).
Atomic Nucleus: At center of the atom, a dense core of tightly packed neutrons and protons with a + charge because of the protons
Rapidly moving electrons form a “cloud” of - charge around the nucleus
Dalton: Atomic Mass Unit (AMU), protons and neutrons 1 each
Mass Number: Total number of neutrons and protons (a)
Atomic Number: Number of protons/electrons, which are the same (b)
Atomic Mass: Exact mass
Isotopes: Different atomic forms of elements
Behave the same way in reactions
Radioactive Isotope: Nucleus decays spontaneously, releasing particles and energy
Half Life: The time it takes for 50% of the parent isotope (original isotope) to decay into the daughter isotope (resulting isotope)
Radiometric Dating: Can use half lives to see how long ago an organism was fossilized or a rock was formed
Energy: Capacity to cause change
Potential Energy: Energy matter possesses from its location to structure
The higher the distance of the electron shell to the nucleus, the greater its potential energy. (+ energy, one shell further, and vice versa)
Valence Electrons: The electrons on the outermost (valence) shell
Orbital: 3D space where electron is found 90% of time
7
Super: 7, sub: 15, N
7, 1
Number of shells, potential energy. Valence electrons, molecules that they can form covalent bonds with.
2.3: The formation and function of molecules and ionic compounds depend on chemical bonding between atoms
Chemical Bonds: Hold attractions between atoms, which share and transfer electrons
Covalent Bond: Two atoms sharing a pair of valence electrons, usually nonmetals
Single Bond: Pair of shared electrons (ex. H—H)
Double Bond: Share 2 pairs of electrons (ex. O=O)
Valence: Bonding capacity of atom, # of atoms needed to complete the shell
Electronegativity: How much atom pulls shared electrons towards itself
Nonpolar covalent bond: Same electronegativity
Polar covalent bond: Electrons not shared equally
Ionic Bond: Cations & anions attract each other
Ions: Charged molecule, from when one atom is so electronegative it rips the electron from its partner
Cation: +
Anion: -
Ionic Compounds/Salts: Compounds formed by ionic bonds
Hydrogen Bond: Noncovalent attraction between hydrogen and electronegative atom
Dipole Dipole: Polar molecules attach to opposite ends like a magnet
Van Der Waals: Adjacent molecules come together close enough that their electron clouds barely touch
London Dispersion Forces: Temporary and very weak
Covalent > Ionic > Hydrogen > Van Der Waals > Dipole Dipole > London Dispersion Forces
Molecular shape is from the positions of an atom’s orbitals and affects function.
Similar shape means there are similar biological effects.
Biological molecules bind temporarily to each other through weak reactions only when their shapes are complimentary
In the diagram, carbon only has 6 valence electrons when it needs 8.
Ionic bonds
You can better understand the molecule’s function and which molecules do similar things
2.4: Chemical reactions make and break bonds
Chemical Reactions: Making and breaking of chemical bonds
Reactants in a chemical equation result in products
Chemical Equilibrium: Number of reactants and products plateaus
All atoms must be accounted for on both sides, and equations are reversible.
Neither, the reaction rate stabilizes and both are pretty much equal
Cellular respiration, used to make ATP from glucose. We inhale oxygen and expel carbon dioxide
Chapter 3: Water and Life
3.1: Polar covalent bonds in water molecules result in hydrogen bonding
Water is polar, since the overall charge is unevenly distributed
Oxygen, δ− Hydrogen δ+
Very fragile hydrogen bonds, so it’s constantly shuffling and reforming
Electronegativity is how strongly an atom pulls electrons towards it, and since oxygen is partially negative, it has a higher electronegativity, which leads to water’s polarity.
Hydrogen bonds with oxygen and vice verse to result in complete valence rings
Hydrogen must bond with oxygen and vice versa
It would be nonpolar, altering the shape and properties
3.2: Four emergent properties of water contribute to Earth’s sustainability for life
Cohesion of molecules
Cohesion: Attraction and sticking together of water molecules
Results in high surface tension (how hard to stretch or break the surface of a liquid)
Helps water and nutrients move upward against gravity in plants
Adhesion: Clinging of one substance to another, helps counter gravity’s downwards pull
Temperature and heat
Kinetic Energy: Energy of motion
Thermal Energy: Total kinetic energy associated with atoms and molecules
Temperature: Average kinetic energy of molecules
Thermal is TOTAL, Temperature is AVERAGE. Not the same!
Heat: Thermal energy transferred from one body of matter to another
Calorie (cal): Amount of heat to raise the temperature of 1g of water by 1°C
Kilocalorie (kcal): Amount of heat to raise the temperature of 1 kilogram (kg) of water by 1°C
Joule (J): 0.239 cal (1 cal = 4.184 J)
Specific Heat: Amount of heat for 1g of the substance ± temperature by 1°C
Heat must be absorbed to break hydrogen bonds, and released when they form
Heat of Vaporization: Amount of heat to turn 1g liquid > gas
Evaporative Cooling: As water evaporates, the surface of the remaining liquid cools down
Contributes to the stability of water temperature in waters & lakes
Prevents organisms from overheating
Floating of ice on liquid water
Water is less dense as a solid than a liquid
Hydrogen bonding makes a crystalline lattice
Holds molecules far apart, making it 10% less dense
Most dense at 4°C
The solvent of life
Solution: Solute (dissolved) + solvent (dissolved in)
Aqueous Solution: Water is the solvent in a solution
Hydration Shell: Sphere of water around a dissolved ion
Hydrophilic: Affinity to water
Hydrophobic: Nonionic, nonpolar, repel water
Colloid: Stable suspension of fine particles in a liquid
Molecular mass: Sum of the mass of all atoms in a molecule
mol(e): Exact # of objects—6.02 x 10²³
Molarity: # moles per solute per Liter of solution
Water adheres to the tree and can “climb” using its high surface tension
The humidity
Idk lol
Its legs would not get wet even when submerged, but if hydrophilic it might absorb the water
3.3: Acidic and basic conditions affect living organisms
Sometimes hydrogen atoms will shift from one molecule to the other, leaving its electron, the hydroxide (OH-) behind and only transferring the hydrogen (H+) ion. This makes a hydronium ion (H3O+)
H+ and OH- are very reactive
Acid: Higher hydrogen ion concentration
Base: Lower hydrogen ion concentration
In an aqueous solution at 25°C, the product of H+ and OH- is always 10-14.
pH = -log[H+]
H+ = 10-pH
Strong acids and bases completely dissociate in water
Most living cells have a pH around 7
Human blood is at 7.4, can’t survive if ± 0.4
Buffer: Substance that minimizes changes in pH
Ocean Acidification: When CO2 dissolves in seawater, reacting with water (making carbonic acid) and lowering ocean pH
Lowers pH and carbonate ion concentration, which is crucial in coral reef formation
105
Chapter 4: Carbon & Molecular Diversity
4.1: Organic chemistry is the key to the origin of life
Organic Chemistry: Study of compounds containing carbon
Major elements to life are C,H,O,N,S,P
Abiotic: Nonliving
Vitalism: There is a life force outside of natural laws
Mechanism: Natural laws create all phenomena
The sparks created a source of energy
4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
Electron configuration determines bonds
Carbon’s shape allows for 4 single bonds
Valence: Number of covalent bonds an atom can form
Hydrocarbons: Organic molecules consisting of only carbon and hydrogen
All of the above in the pictures
Isomers: Same number of atoms of the same elements but different structures (so different properties)
Structural Isomers: Different covalent arrangements of their atoms
Cis-Trans Isomers/Geometric Isomers: Carbons have covalent bonds to the same atoms, but they differ in spatial arrangements
Enantiomers: Isomers that are mirror images of each other
Can’t
Butane and 2-Mehtopropane
Lots of hydrocarbon chains
4.3: A few chemical groups are key to molecular function
Properties also depend on molecular components
Functional group: Chemical group that is directly involved in chemical reactions
Hydroxyl Group: —OH, polar and forms h bonds with water, ends in -ol
Carbonyl Group: C=O, Ketone (sugars with these are ketoses) or aldehyde (sugars with these are aldoses)
Carboxyl Group: —COOH, acts as acid, can donate H+ because covalent bond between oxygen and hydrogen is so polar
Amino Group: —NH2, acts as base, can pick up H+ from surrounding solution
Sulfhydryl Group: —SH, two can react and form a “cross link” to stabilize protein structure
Phosphate Group: —OPO32-, contributes negative charge of -1 when in a chain of phosphates, -2 at the end
Methyl Group: —CH3, affects expression of genes when bonded to DNA or proteins that bind to DNA
Adenosine Triphosphate (ATP): 3 phosphate string + adenosine, when it loses a P it becomes ADP
Stores potential to react with water or other molecules
Not sure
It releases a phosphate group
Can’t draw
Chapter 5: Structure & Function of Large Biological Molecules
5.1: Macromolecules are polymers, built from monomers
Macromolecules: Large carbohydrates, proteins, and nucleic acids
Polymers: Long chain like molecule consisting of many similar building blocks linked by covalent bonds (picture a chain of boxcars)
Monomers: The building blocks of polymers
Enzyme: Specialized macromolecules which speed up chemical reactions
Condensation Reaction: The reaction that connects a monomer to a monomer or polymer, where two molecules are covalently bonded with the loss of a small molecule
Dehydration Reaction: Water molecule as byproduct, two molecules covalently bonded by its loss

Hydrolysis: The reverse of dehydration synthesis, bond between monomers is broken by addition of a water molecule

Carbohydrates, lipids, proteins, nucleic acids. Lipids aren’t polymers since they don’t have a monomer unit
9
Hydrolysis breaks the food down, dehydration fuses it with you
5.2: Carbohydrates serve as fuel and building material
Carbohydrates: Sugars and polymers of sugars
Monosaccharides: Simple sugars, monomers of more complex sugars
Molecular formula is a multiple of CH2O
Either an aldose or ketose. Also count number of carbons, 3-7
Triose, pentoses, and hexoses are most common
Disaccharide: 2 monosaccharides joined by glycoside linkage
Glycoside Linkage: Covalent bond formed between two monosaccharides by a dehydration reaction
Must be broken down into monosaccharides to be used for energy by organisms
Polysaccharide: Macromolecules formed by monosaccharides, used as storage material and structural building material
Starch: Polymer of glucose monomers, stored as plastids
Plastids: Granules within cellular structures (including chloroplasts)
Extra sugar can be withdrawn by hydrolysis
Most of glucose monomers in synarchy joined by 1-4 linkages. Simplest form (amylose) is unbranched, more complex is branched
Glycogen: Polymer of glucose, more extensively branched than amylopectin
Cellulose: Polysaccharide, major component of cell walls
Two slightly different ring structures for glucose, alpha (a) and beta (b)
In starch, all a, so helical, efficiently stores glucose units
In cellulose all b, so it is straight and never branched. The h bonding between parallel cell walls hold them together
Microfibrils: Units in which parallel cellulose molecules are held together and grouped
Chitin: Carbohydrate used by arthropods to build their exoskeletons
First leathery and flexible, but is hardened when the proteins are chemically linked to each other or encrusted with calcium carbonate. b linkages
C3H6O3
C12H22O11
Adds helpful gut bacteria back in
5.3: Lipids are a diverse group of hydrophobic macromolecules
Lipids: Hypdrophobic, the one class of large biological molecules that does not include true polymers, not macromolecules
Fat: A glycerol (alcohol) joined to three fatty acids
Fatty Acid: Long carbon skeleton, 16-18 carbon atoms in length. Carbon at the end is part of a carboxyl group
(Relatively) nonpolar C—H bonds in hydrocarbon chains of the fatty acids are why the fats are hydrophobic
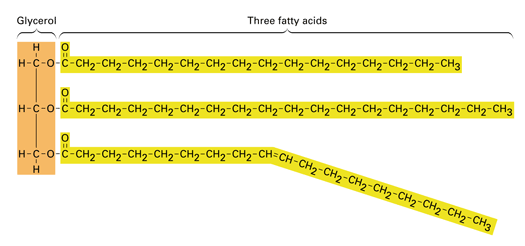
Fatty acid is joined to glycerol by dehydration synthesis, resulting in an ester linkage
Ester Linkage: Bond between hydroxyl and carboxyl group
Saturated Fatty Acid: No double bonds between carbon atoms composing a chain, meaning lots of hydrogen and it is solid at room temperature
Unsaturated Fatty Acid: 1+ Double bonds, one fewer hydrogen atom on each double bonded carbon
Hydrogenated: Added hydrogen to unsaturated fatty acid to make it solid at room temperature
Trans Fats: Unsaturated fats with trans double bonds
Fats are used for energy storage, since 2 gram has >2x the amount of energy as a gram of a polysaccharide (such as starch)
Also cushions vital organs and insulates the body
Phospholipid: Make up cell membranes, 2 fatty acids + glycerol, 3rd joined to a phosphate group, - charge
Hydrophobic tail, hydrophilic head
Steroids: 4 fused rings, different steroids alter different chemical groups
Cholesterol: Animal cell membrane component, precursor of many other steroids
3 lipid tails, and glycerol base, in phospholipids there are two tails and glycerol base + phosphate group
Because sex hormones are steroids which are lipids
Like a circle of single layered phospholipids around the oil droplet, with the tails facing the oil side
5.4: Proteins include a diversity of structures, resulting in a wide range of functions
Enzyme proteins act as catalysts to regulate metabolism, which speed reactions without being consumed
Proteins are all connected by the same set of 20 amino acids
Peptide Bond: Bond between amino acids
Polypeptide: Polymer of amino acids
Polypeptide Backbone: Repeating sequence of atoms (in purple in diagram)
Protein: Biologically functional molecule with 1+ polypeptides, folded and coiled into a 3D structure
Nonpolar: Glycine, Alanine, Valine, Leucine, Isoleucine, Tryptophan, Proline, Phenylalanine, Methionine
Proline: Causes kinks in alpha-helices due to rigid ring structure, often found in turns
Glycine: Smallest, increases flexibility, often found in turns
Methionine: Start codon (AUG) in translation
Tryptophan: Largest, precursor for serotonin
Polar Uncharged: Serine, Cysteine, Threonine, Tyrosine, Glutamine, Asparagine
Cysteine: Forms disulfide bonds (covalent), stabilizes protein structure
Tyrosine: Can be phosphorylated, precursor for dopamine & thyroid hormones
Polar Positive: Lysine, Arginine, Histidine
DNA
Polar Negative: Aspartic Acid, Glutamic Acid
Amino Acid: Organic molecule with amino and carboxyl group
Proteins are 50%+ of dry mass of most cells
Primary Structure: Unique sequence of amino acids
Secondary Structure: Coils (a helix) or folds (b pleated sheet)
Tertiary Structure: Overall shape of polypeptide from interactions resulting from side chains (R Groups)
Hydrophobic Interaction: Exclusion of nonpolar substances by water molecules which lead to
Primary has peptide bonds
Secondary has hydrogen bonds
Tertiary has mainly disulfide bonds with some hydrogen bonds
Quaternary has electrostatic bonds with some hydrogen bonds
Electrostatic Bond: One atom loses an electron, one gains one
Hydrogen Bonding
5.5: Nucleic acids store, transmit, and help express hereditary information
Gene: Programs amino acid sequences of polypeptides
Nucleic Acid: Polymer made of monomers called nucleotides. DNA and RNA
Deoxyribonucleic Acid (DNA): Provides directions for own replication, directs RNA synthesis, and RNA controls protein synthesis (gene expression)
Ribonucleic Acid (RNA): A nucleic acid that carries instructions from DNA to control synthesis of proteins, directs production of polypeptides
Polynucleotides: Nucleic acids, consist of monomer nucleotides
Nucleotide: Three parts, a 5 carbon sugar (pentose), nitrogenous base, and 1-3 phosphate groups
Two types of nitrogenous bases
Pyrimidine: One 6 membered ring of carbon and nitrogen atoms
CUT
Purines: Larger than pyrimidines, six membered ring fused to a 5 member ring
AG (Pure as gold)
In DNA, the sugar is deoxyribose, in RNA, it’s ribose
Sugar atoms have a prime symbol (‘) after a number
Double Helix: Shape of DNA molecule
Antiparallel: The sugar phosphate backbones in DNA go from 5’ → 3’
5.6: Genomics and proteomics have transformed biological inquiry and applications
Bioinformatics: Use of computers and tools to analyze large datasets
Genomics: Analyzing large sets of genes and sometimes comparing genomes to other species
Proteomics: Protein sequences are determined using either biological techniques or translating DNA sequences