Wavelength and Frequency
Formulas:
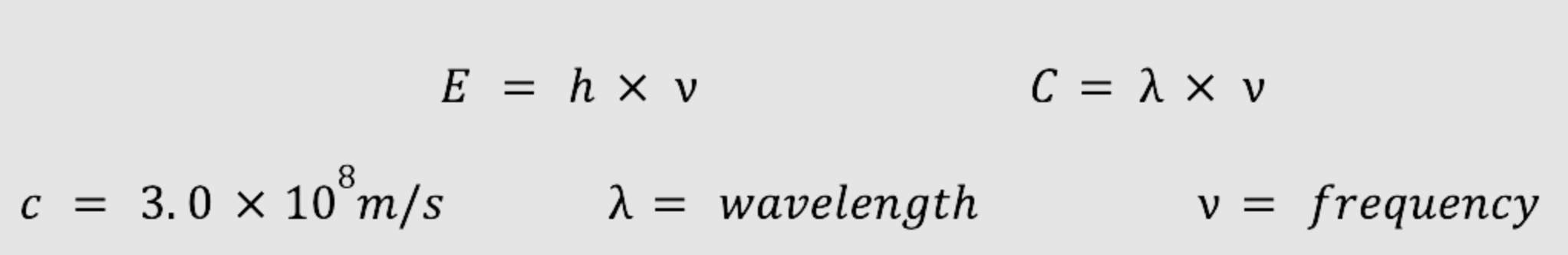 Practice problems:
Practice problems:
What is the frequency of a photon whose energy is 6.00 x 10-15 J? Identify the color of this radiation.
What is the energy of a photon whose frequency is 2.22 x 1014 s-1?
What is the wavelength of radiation whose frequency is 5.00 x 1012 MHz?
Principles/ Rules
Aufbau Principle: Lower energy levels must be filled before higher ones
Pauli Exclusion Principle: Electrons in the same orbital must have opposite spins
Hund’s Rule: One electron must be present before pairing
Periodic Trends

Periodic Table
Complete the following table based on the information given.
Isotopic Notation |
Nucleotide Symbol | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
He-4 | 42He | 2 | 4 |
| 2 | 2 |
Mg 2+-____ | | 12 | 24 |
| | |
Zn-65 | | 30 | |
| | 30 |
Br 1-- 80 | | |
| 35 | | |
Al3+-_____ | | |
| 13 | 14 | |
C 4--_____ | | 6 |
| | 8 | |
Si-_____ | | | 29 |
| | 14 |
 Note
Note Studied by 98 people
Studied by 98 people Note
Note Studied by 19 people
Studied by 19 people Note
Note Studied by 23 people
Studied by 23 people Note
Note Studied by 15 people
Studied by 15 people Note
Note Studied by 19 people
Studied by 19 people Note
Note Studied by 16 people
Studied by 16 people Knowt
Knowt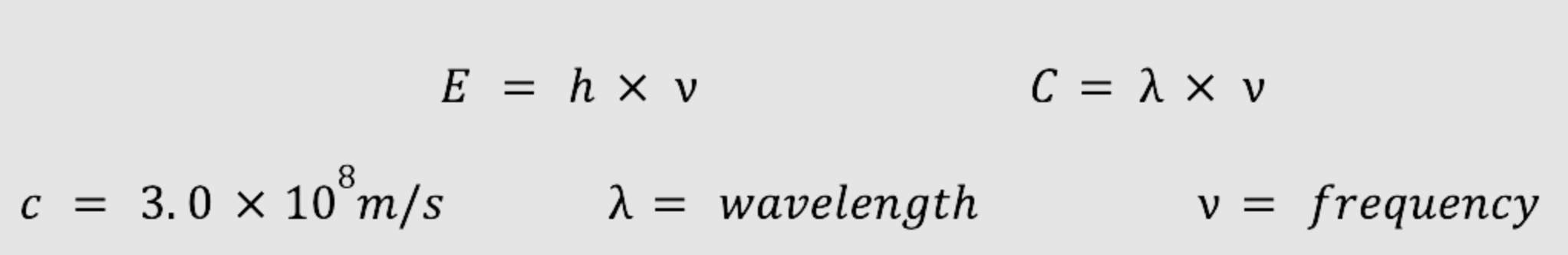 Practice problems:
Practice problems: