Fluorescence spectroscopy
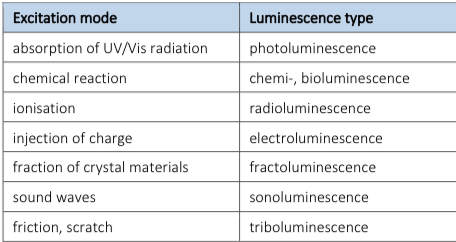
Luminescence
Light is not generated by high temperatures so molecules can be excited in different ways, this phenomenon is “cold emission”.
Excitation by stimulation where some of the excitation energy is emitted in form of electromagnetic waves to the environment, this phenomenon is ==Luminescence==. Substances created are luminophores.
Types of luminescence
Fluorescence is when emitted light is at a higher wavelength than excitation light.
Jablonski schematic
Energy levels (S0, S1, …) split into vibrational levels (from the vibrating movements of the molecule); each vibrational level splits into rotational levels (from the rotation around an axis of the molecule).
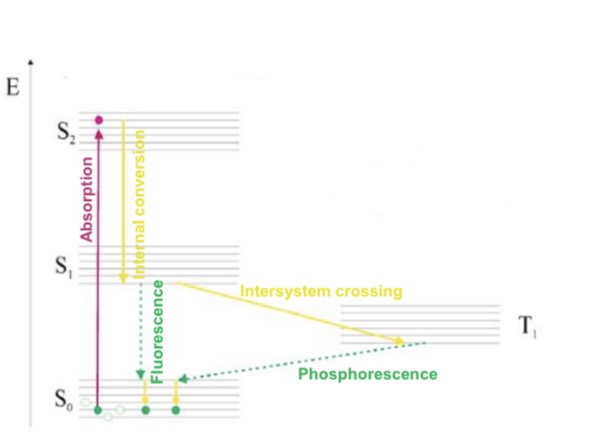
Transition from ground state S0 to excited state S1,S2… is very fast. Duration is femtoseconds(fs=10^-15s).
Thermal relaxation when the excited electron is set to the lowest excitation level S1. Duration is picoseconds(ps=10^-12s).
Unstable excited state so to return to ground state:
a. Non-radiative transition(internal conversion)
b. Transition with radiation(luminescence)
I. ==Fluorescence==; electrons return from lowest excited state(S1) to ground state in one step, duration is ns = 10^-9s.
II. ==Phosphorescence==; spin turns from singlet(S1) to triplet(T1) which is ==intersystem crossing== then energy returns from lowest excited state(T1) to ground state. Duration is 10^-6s.
Pairing of electron spin
Conditions: Multiplicity (M) is the number of possible orientation states of the magnetic moment assigned to the spin state (S) relative to the directions of the magnetic field, M= 2S+1.
It can be;
• Singlet state - This confirms the Pauli exclusion principle by permitting transition,
when S = 0 and M = 1
• Triplet state - This denies Pauli exclusion principle by forbidding transition,
when S = 1 and M = 3
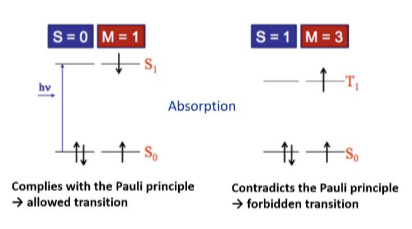
Photo emission happens during transition of the lowest vibrational level(S1) of first excited state, this is ==Kasha’s rule.==
Proof of Kasha’s rule;
If change is made to the excitation wavelength in the excitation range the emission spectra shape will not change.
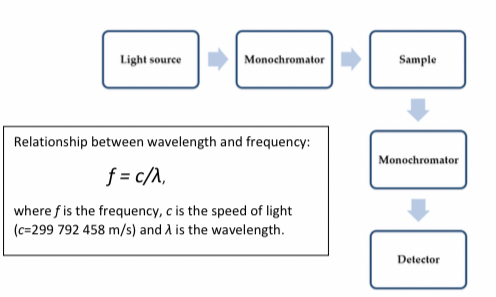
Fluorimeter
It is used to measure fluorescence, the arrangement of the second monochromator perpendicular to to sample solution is because excitation light reaching the sample can reach the detector and affect results if in linear arrangement but in the perpendicular arrangement we can exclude the light.
Concept of excitation and emission spectrum
Spectrum is the dependence of intensity on wavelength.
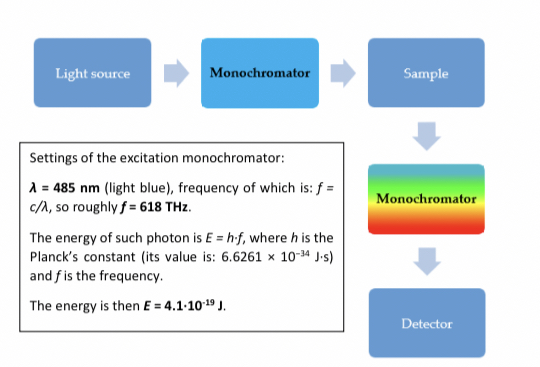
Checking properties of a fluorophore by estimating emission wavelength(in emission Monochromator) and then setting the range for excitation wavelength in the excitation site that produces the highest emission. This is the ==excitation spectrum==. The graph of this will be shifted towards the left which is the wavelength in UV range.
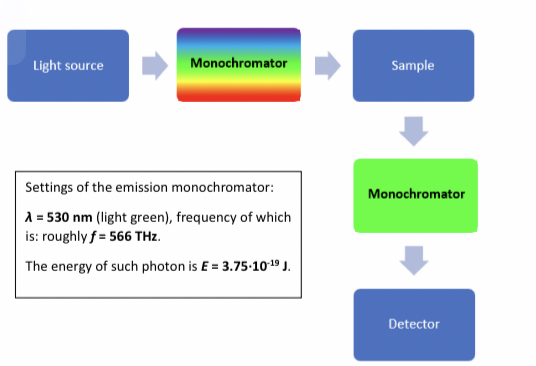
* The light green Monochromator is the emission side.
If excitation wavelength is constant as a function of emission wavelength the ==emission spectrum== is formed. The graph will be shifted to the right in the Infrared range.
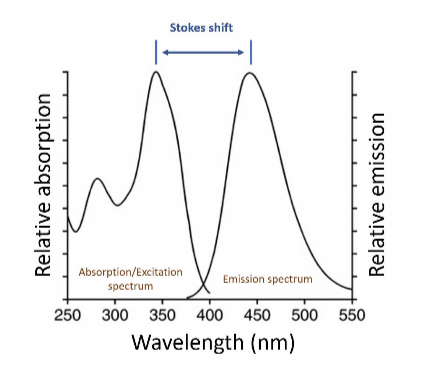
Stokes shift
The excitation and emission spectrums are mirror images of each other and the gap between the difference in their wavelengths is called the ==stokes shift.==
This can also be seen on the Jablonski diagram, luminescence is preceded by internal conversion or vibrational relaxation so luminescence has higher wavelength and energy than absorption energy.
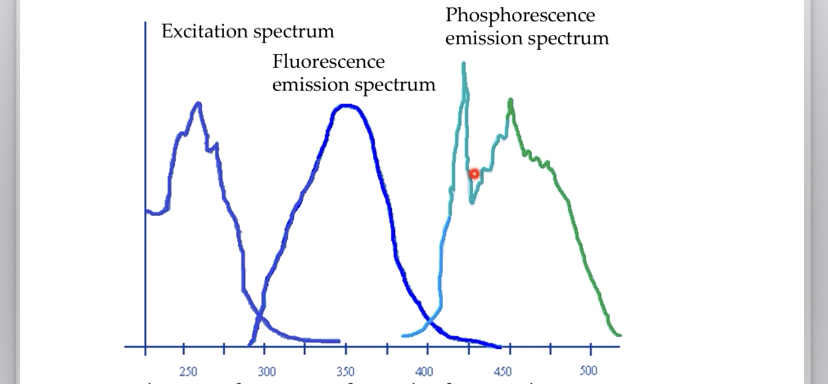
==Quantum yield of luminescence== is the quotient of the number of emitted and absorbed photons. It specifies the efficiency with which the molecule converts excitation into emission.
Its values are between 0 and 1.
Q =N(emit)/N(abs)
N(emit) is number of photons emitted and N(abs) is number of photons absorbed.
Q = K(fluor)/K(sum)
K(fluor) is fluorescence K(sum) added probability of all radiative and non radiative transitions.
==Luminescence lifetime== is the time elapsed between excitation and emission. Its value in the
case of fluorescence is some ns (1 ns = 10-9s). In the case of phosphorescence, the lifetimes
are much longer.