Clinical Chem Acid-Base Balance & Electrolytes
Learning outcome
- Explain the role of buffer systems in maintaining acid-base balance and pH
- Explain the role of buffer systems in regulating pH of the intracellular fluid and extracellular fluid
- To understand the concept of fluid and electrolyte balance
- To understand homeostasis of selected electrolytes and related disorders.
Key Terms
Acid - proton (H+) donor
Strong acid - low affinity between acids & protons; highly dissociated in aqueous solution
Weak acid - high affinity between acids & protons; poorly dissociated in aqueous solution
Base - proton (H+) acceptor
Alkali - a base that is soluble in water & produces hydroxyl ion (OH-)
Concepts
Acid-base balance
- a state of equilibrium between acidity & alkalinity of the body fluids
- mechanisms of our body to maintain the body fluids close to neutral pH to ensure proper physiological functions
- measured using the pH scale
pH
- used to determine the acidity or alkalinity of a fluid
- measure the hydrogen ion (H+) concentration relative to that of a given standard solution
- negative logarithm of H+ concentration
pH = -log [H+]
- a scale from 0-14
- neutral (pH = 7.0)
- acidic (pH < 7.0)
- Basic/alkaline (pH > 7.0)
- very slight change in pH will have disastrous effects on cells & tissues
- acid-base balance is regulated within a narrow range for normal physiological functions
- normal blood pH: 7.35 - 7.45
- acidosis (acidemia)
- blood has low pH of less than 7.35
- overproduction of acid or excessive loss of bicarbonate or buildup of carbon dioxide
- metabolic or respiratory acidosis
- alkalosis (alkalemia)
- blood has high pH of greater than 7.45
- over-abundance of bicarbonate or a loss of acid or a low level of carbon dioxide
- metabolic or respiratory alkalosis
- Different mechanisms to regulate and maintain the blood pH within the fairly narrow optimum range
- Involve:
- lungs
- kidneys
- chemical buffer systems
Buffers
- solutions that can resist significant changes in pH
- maintain stable [H+] in biological systems
- consist of a conjugate acid-base pair
- present in both intracellular & extracellular fluids
- finite buffering capacity
- most common buffer systems:
- bicarbonate buffer system
- phosphate buffer system
- protein buffer system
]]Bicarbonate buffer system]]
Major extracellular buffer system operates in both lungs and kidneys
maintain pH homeostasis of the blood
consists of carbonic acids (H2CO3) as the weak acids and its conjugate bases, bicarbonate ions
H2CO3 is formed when dissolved carbon dioxide combines with the water in the bloodstream
↑ [H+] - HCO3- will accept H+ to form H2CO3
↓ [H+] - H2CO3 will donate H+ and turn in to HCO3-
Compensation for the pH:
lungs → decrease the carbonic acids level through exhalation
→ adjust the respiration rate to decrease or increase the CO2
kidneys → reabsorbs bicarbonate ions or regenerate new bicarbonate ions
→ produce more acidic or more alkaline urine
]]Phosphate buffer system]]
- important in buffering renal tubular fluid and intracellular fluid
- comprised of hydrogen phosphate ions & dihydrogen phosphate ions
- Hydrogen phosphate ion is freely filtered through glomerulus → high concentration intracellularly & in urine
- critical renal & urinary buffer → allow secretion of H+ ions from the tubular cells in conjuction with the generation of HCO3-
- ↑ [H+] - HPO4(2-) will accept H+ to form H2PO4-
- ↓ [H+] - H2PO4- will donate H+ and turn in to HPO4(2-)
- Catalysed by enzyme carbonic anyhydrase
]]Protein buffer system]]
- most abundant and important buffer system in the body fluids
- either intracellular or extracellular
- protein molecule carries both basic and acidic groups → acts as proton (H+) acceptor or donors
- Haemoglobin (Hb) is the major intracellular buffer system
Lungs
- HbO2 is formed from HHb by releasing H+ which will react with HCO3 and form H2CO3 + CO2 + H2)
- the CO2 is then eliminated by exhalation
Tissues
- CO2 produced by metabolism enters the blood, hydrated to form H2CO3
- the H2CO3 ionises to form H+ & HCO3-
- HbO2 accepts the H+ to form HHb
Disorders of Acid-Base Balance
- imbalances in acid-base equilibrium
- respiratory acid-base disorders
- caused by ventilatory dysfunction
- a change in the pCO2
- metabolic (non-respiratory) acid-base disorders
- a change in the bicarbonate level
- resulting from a change in renal or metabolic functions
Respiratory Acidosis
- decreased alveolar ventilation (hypoventilation) leads to a decrease in the elimination of CO2 from the lungs
- Possible causes:
- ineffective removal of CO2 from the blood in lung diseases
- trauma, infection or inflammation of central nervous system
- drugs (e.g., barbiturates, morphine) % alcohol
- congestive heart failure → decreased cardiac output
- Laboratory findings:
- pH < 7.35
- ↑ pCO2
- normal bicarbonate concentration
- acute respiratory acidosis: pH drops 0.1 unit for every 15 mmHg increase in pCO2
- chronic respiratory acidosis: pH drops 0.05 unit for every 15 mmHg increase in pCO2
- Compensatory mechanisms
- via the haemoglobin and protein buffer systems
- through metabolic processes in kidneys
- ↑ excretion of H+
- ↑ reabsorption of HCO3-
- ↑ formation of ammonia
- via respiratory organs (if functional)
- ↑ rate & depth of breathing
Respiratory Alkalosis
- Results from an increased rate or depth of breathing or both
- excessive elimination of CO2 by the lungs/deficit in pCO2
- Possible causes:
- hypoxemia- & hysteria-induced hyperventilation
- deugs, e.g., nicotine & salicylates
- pulmonary emboli & pneumonia
- gram-negative septicemia, meningitis or encephalitis
- Laboratory findings:
- ph > 7.45
- ↓ pCO2
- normal bicarbonate concentration
- Compensatory mechanisms
- haemoglobin & protein buffer systems
- kidneys excrete more HCO3 in urine
Metabolic Acidosis
- ↓ HCO3- level (< 24 mmol/L)
- Possible causes:
- direct administration or ingestion of acid-producing substances (e.g., ammonium chloride, calcium chloride, ethanol)
- production of organic acids (e.g., in diabetic ketoacidosis and lactic acidosis)
- reduced excretion of acids (e.g., renal tubular acidosis0
- excessive loss of HCO3- from diarrhea
- Laboratory findings:
- pH < 7.35
- ↓ pCO2
- ↓ bicarbonate concentration
- normal or increased anion gap
- Compensatory mechanisms
- via respiratory mechanisms (i.e., quick & shallow breathing)
- by kidneys (similar to those occur in respiratory acidosis)
Metabolic Alkalosis
- an excess or gain in HCO3-
- possible causes:
- increase in bases (i.e., massive blood transfusions, infusion of intravenous solution high in HCO3-, ingestion of large quantities of antacids)
- decreased excretion of bases (i.e., prolonged use of diuretics)
- loss of acidic fluids (i.e., prolonged vomiting, upper duodenal obstruction, cystic fibrosis)
- laboratory findings:
- pH > 7.45
- normal pCO2
- ↑ bicarbonate concentration
- compensatory mechanisms
- via respiratory system to retain CO2 (i.e., slower & depper breaths)
- by kidneys (excrete > HCO3- & form < NH3)
Fluid & Electrolytes
Average water content: 40% - 75% of total body weight
- ~60% in men, ~55% in women
Located in intracellular & extracellular compartments
- intracellular fluid (ICF)
- extracellular fluid (ECF)
- intravascular ECF (plasma)
- interstitial cell fluid
Movement of water & distribution of water in different body fluid compartments are
- determined by osmolality & colloid osmotic pressure
- controlled by maintaining the concentration of electrolytes and proteins
Electrolytes are charged atoms or molecules found kn body fluids that are important for
- regulation of water distribution, osmotic pressure, cell permeability
- nerve transmissions to muscles
- oxidation-reduction reactions, maintenance of blood pH
May be classified as:
- anions (negatively charged)
- cations (positively charged)
Important physiologic electrolytes
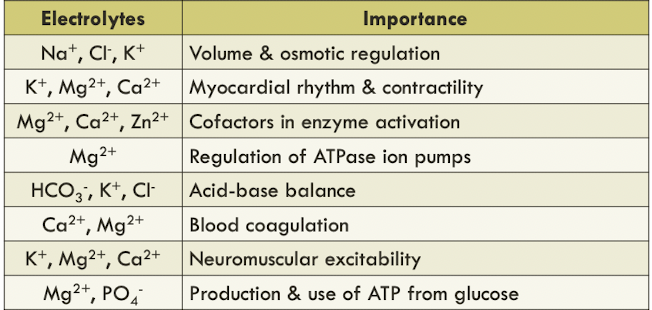
sodium (Na+), potassium (K+), chloride, (Cl-) & bicarbonate (HCO3-) occur primarily as free ions
- known as electrolyte profile
40% of calcium (Ca2+) & magnesium (Mg2+) are bound by proteins
Electrolyte balance - the quantities of electrolytes gained is equal to those it loses
Electrolyte imbalance is life-threatening
Anion gap - the difference between the unmeasured anions and the unmeasured cations
Calculation of anion gap
- determine certain types of electrolyte disorders
- as a marker of quality control of electrolyte testing
- a trend of increased or decreased anion gap in a run of patient specimens may indicate consistent testing errors in one or more electrolytes
Selected Electrolytes & Disorders
Sodium (Na+)
- Major cation in extracellular fluid (~90%)
- main source: sodium containing food additives, e.g., table salt, monosodium glutamate
- excess sodium is excreted in the urine or through sweating
Regulation
- depends on the intake & excretion of water, and renal regulation of Na+
- 3 primary processes:
- intake of water
- excretion of water
- excretion of Na+ through aldosterone, angiotensin II & atrial natriuretic peptide (ANP)
- 2 major homeostatic systems
- renin-angiotensis-aldosterone (RAA) system
- antidiuretic hormone (ADH)
- stimulated via:
- hypovolemia
- hypotension
- decreased renal perfusion
- hyperosmolality
Clinical Significance
- hyponatremia
- serum/plasma level of Na+ <135mmol/L
- caused by:
- increased Na+ loss (e.g., prolonged vomiting, diuretic use, severe burns)
- increased water retention (e.g., renal failure, hepatic cirrhosis, congestive heart failure)
- water imbalance (e.g., excess water intake)
- classification based on serum/plasma osmolality (ECF volume)
- low osmolality (e.g., ↑sodium loss, ↑water retention)
- normal osmolality (e.g., severe hyperkalemia, hyperproteinemoa)
- high osmolality (e.g., hyperglycaemia, mannitol infusion)
- acute hyponatremia (<48 hr); chronic hyponatremia (longer period)
- pseudohyponatremia
- an uncommon artifact results from in vitro hemolysis during blood sample processing in the laboratory
- a decrease of serum [Na+] but normal serum osmolality
- treatment aims to correct the underlying causes
- conventional treatment:
- fluid restriction
- hypertonic saline and/or other pharmacologic agents (i.e., AVP receptor antagonist)
- possible complications:
- osmotic demyelination syndrome
- cerebral edema
- Hypernatremia
- ↑serum level of Na+
- serum [Na+] > 160mmol/L has mortality rate of 60-75%
- caused by:
- excess loss of water relative to Na+ loss
- decreased water intake
- increased Na+ intake or retention (i.e., excess ingestion of salt)
- symptoms:
- altered mental status
- lethargy
- irritability & restlessness
- seizures
- muscle twitching & hyperreflexes
- fever
- nausea/vomiting
- difficult respiration & increased thirst
Chloride (Cl-)
- Major anion in extracellular fluid
- involved in maintaining osmolality, blood volume & electric neutrality
- filtered out by glomerulus & passively reabsorbed by the proximal tubules
- excess chloride is excreted in the urine and sweat
- Maintain electrical neutrality
- reabsorption of Na+ along with Cl- in proximal renal tubules
- Cl- acts as rate-limiting factor or
- through chloride shift
Clinical Significance
- Hypochloremia
- decreased level of Cl- in plasma
- due to prolonged vomiting, diabetic ketoacidosis, aldosterone deficiency, pyelonephritis
- conditions associated with high serum [HCO3-]
- Hyperchloremia
- increased level of Cl- in plasma
- caused by dehydration, renal tubule acidosis, prolonged diarrhea & diabetes insipidus
- excess loss of HCO3-