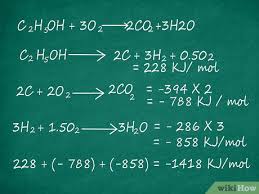Unit 5 - Reaction Energy
LANGUAGE DEVELOPMENT
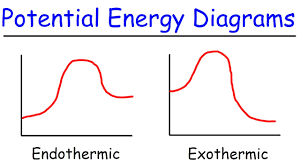
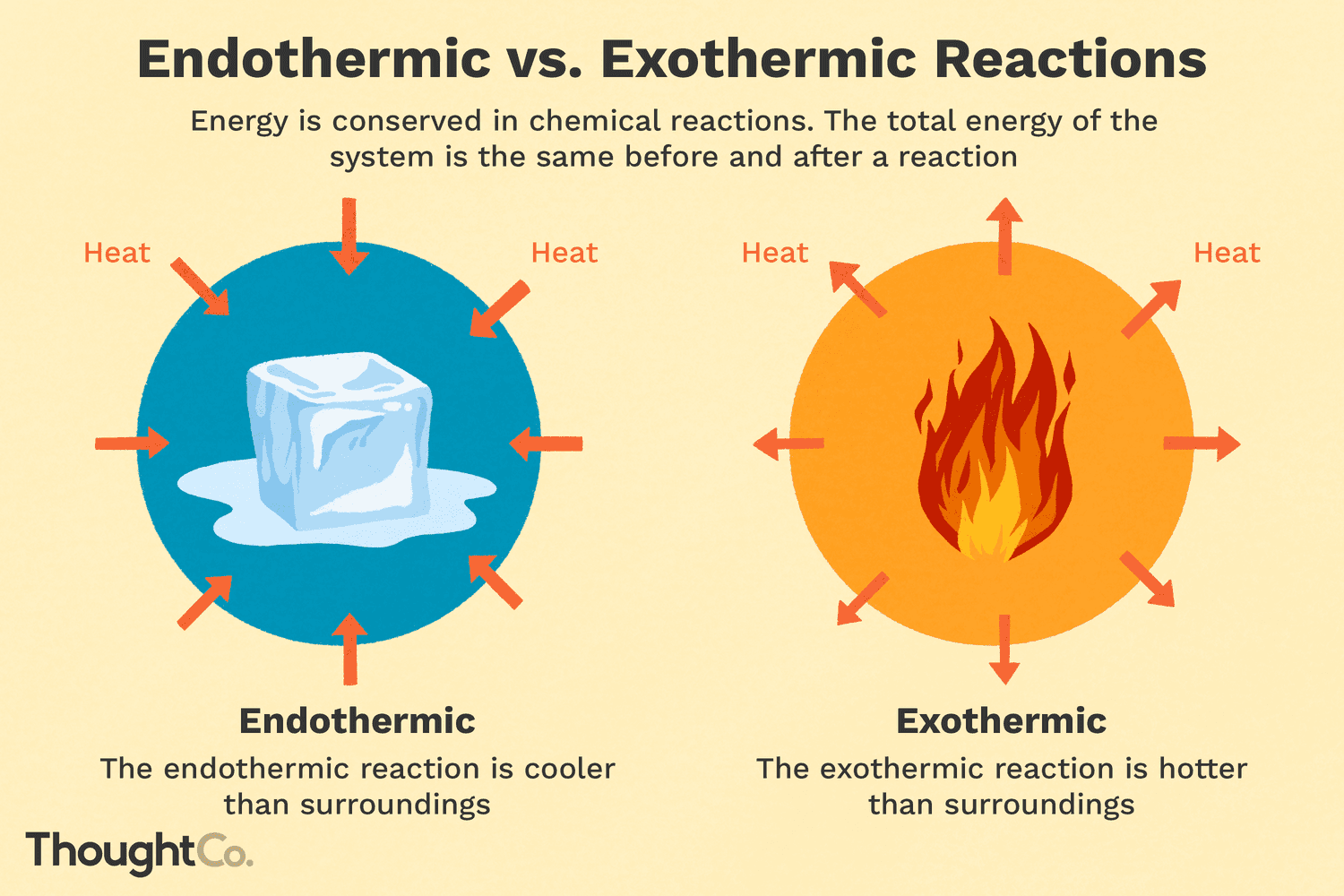
exothermic reaction - releases energy (heat/light/electricity)
endothermic reaction - absorbs energy in the form of heat
enthalpy - energy changes that occur during a reaction —> total heat content in a system
hess’s Law - if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the sum of the ΔH values for each step
collision theory - in order for a chemical reaction to occur, particles must collide
activation energy - the minimum amount of energy needed to start a chemical reaction
catalyst - changes the rate of a chemical reaction without being consumed during the reaction
reaction rate - the speed at which a chemical reaction takes place
5.1 - Energy in Chemical Bonds
exothermic reaction (-)
releases energy in the form of:
heat
light
sound
decrease in temperature
endothermic reaction (+)
absorbs energy in the form of:
heat
enthalpy
energy changes occuring during a reaction
change in enthalpy —> energy released or absorbed as heat by a system
constant pressure
not directly measured
equation: H(products) - H(reactants)
kJ/mol
extensive property - depends on the energy of a substance’s particles
thermochemical equation - includes the value of change in H —> right side of the equation
physical states of reactants and products
adding energy to the equation
standardize enthalpy values:
STP (standard temperature and pressure) —> 25 degrees Celsius (298 K) and 1 atm
formation of 1 mole of the substance
burning different types of fuels/combusting them
synthesis reactions - compound is produced from pure elements
BOTH EQUATIONS MUST RESULT IN THE SAME AMOUNT OF PRODUCTS
calorimeters
well-insulated —> minimize energy transfer
constant pressure
measures change in enthalpy under constant pressure
chemical reactions
energy transfer between 2 substances
measure temp before/after reaction to determine energy flow
bomb calorimeter
combustion
large amounts of gaseous products
large changes in thermal energy
contained at fixed volume —> increased pressure
enthalpy of a reaction
heat absorbed at constant pressure —> extensive
Hess’s Law: Below
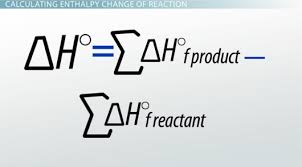
Hess’s Law is based on the law of conservation of energy
pure carbon
graphite vs diamond —> graphite is standard and diamond is highly pressurized at a hot temperature
graphite: enthalpy is 0
diamond: can’t be measured because its not at constant pressure
assumes energy difference between reactants/products is independent of the route taken to get from one to the other
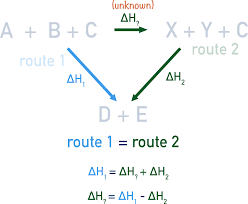
calculating enthalpy of a reaction
bond energies
endothermic - breaking bonds
total bond energy is greater in the reactants
exothermic - forming bonds
total bond energy is greater in the products
predict the stability of the products of a reaction
less bond energy = more potential energy
less potential energy = a more stable reaction
computational chemist
use computer simulations to solve chemical problems
mathematical models
examine relationships among chemical reactions
how atoms interact with reactant concentrations, wind, air, temperatures, and seasonal changes in sunlight
sub microscopic level
improve productivity/efficiency of industrial processes
predict how reacting molecules combine under different conditions
super computers and computing clusters —> require massive amounts of data
5.2 - reaction rates
collision theory - particles must collide for a reaction to occur
break bonds in reactants
get the different particles to interact
correct orientation/enough energy —> effective
increase reaction rates
concentration
increased = total number of effective collisions increases
low: one reaction
medium: 4 reactions
high: more than 20 reactions
temperature
particles have more energy and the temperature increases
surface area
when phases come together (area of contact)
heterogenous reaction
reaction rate depends on surface area
nature of reactants
different substances vary greatly in their tendencies to react
chemical structure
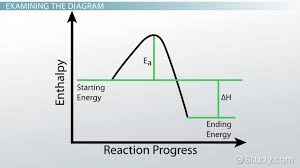
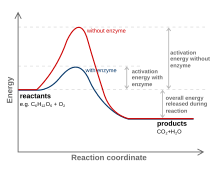
activation energy - hill
higher than reactants and products
transition states —> activated complex
brief existence
higher frequency of collisions —> more energy to form activated complex
calculating energy requirements
Change in E forward: energy of products - energy of reactants
Change in E reverse: energy of reactants - energy of products
Ea: energy of activated complex - energy of reactants
Ea’: energy of activated complex - products
lowering activation energy
catalyst - substanec changing the rate of a chemical reaction without being consumed during the reaction
don’t appear in final reaction or transitioned into another state or combined with another element
lower activation energy = required to start the reaction
photosynthesis and cellular respiration
proceed rapidly at relatively low temperatures
catalyzing changes
first catalyst speeds up a reaction
removes hydrogen atoms from long chains of carbon atoms
double bonds between carbon/polyetheline molecules
react more with other compounds
reduces activation energy and breaks apart plastic at the double bonds —> hydrocarbon chains at various lengths
short enough to be recycled
expensive —> recyclable/reusable
rate law - expresses dependence of reaction rate on the concentration of the reactants
R = k [A]^n * [B]^m
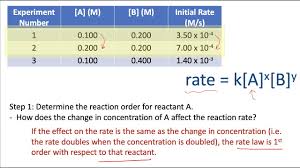
concentration/reaction rate
chemical kinetics
to study how fast chemical reactions happen
what affects this speed
measures:
reaction rate
concentration changes over time
how rate depends on concentration, temperature, and catalysts
predicts: how long a reaction takes to occur
reaction efficiency
designing safer/faster processes for industrial explosives