Principles of Chemistry
The Three States of Matter
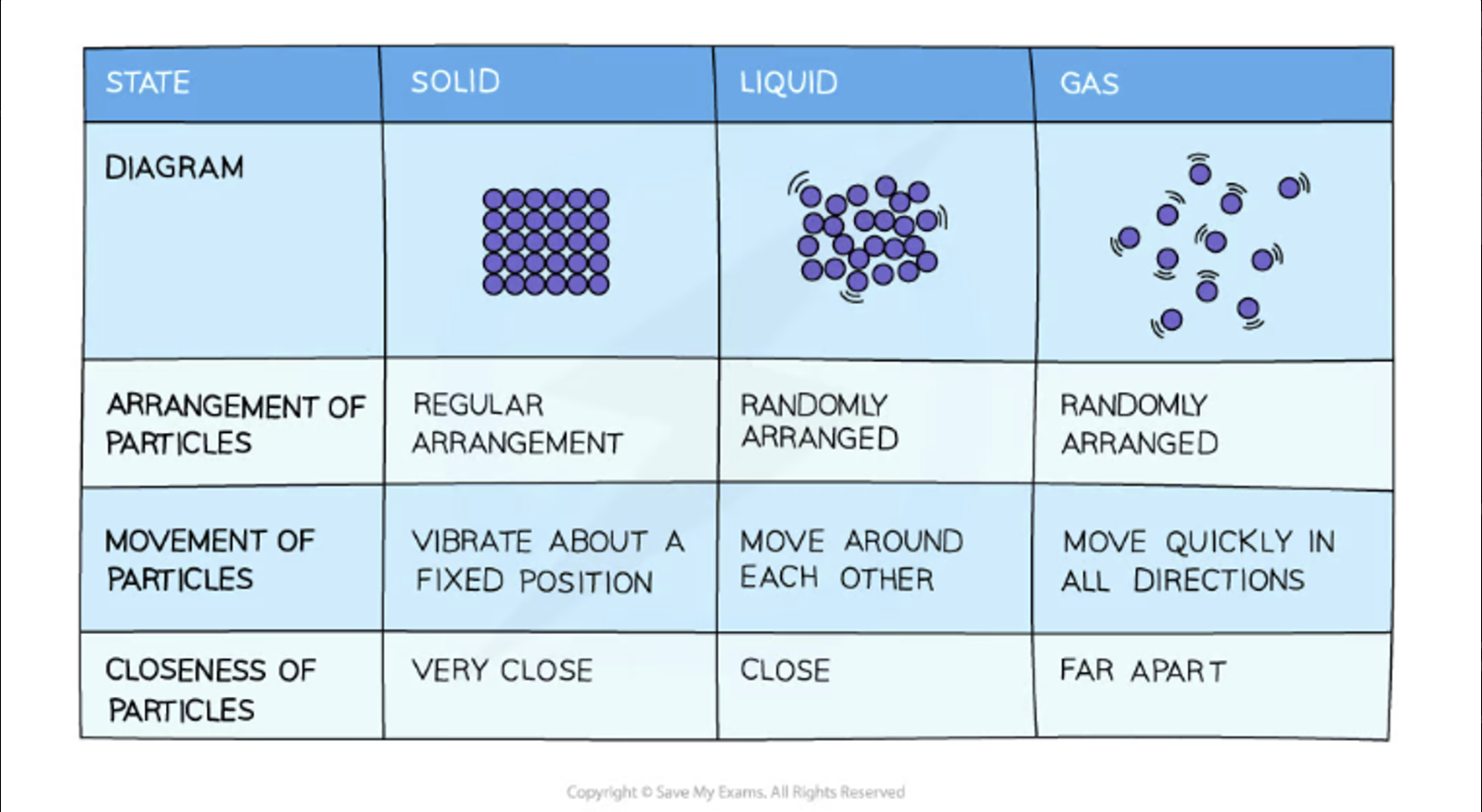
Properties of the States of Matter
Properties of Solids
Regular arrangement
They DONT move, they vibrate.
They are not fluids and cannot be compressed.
Particles are very dense(closely packed).
Low energy between particles
Properties of Liquids
Irregular arrangement
The particles slide past each other
Can be compressed
Particles are close
Medium energy between particles
Fluid
Properties of Gases
Random arrangement
Move quickly and randomly in all directions
Can be compressed
Fluid
Particles are far
High energy between particles
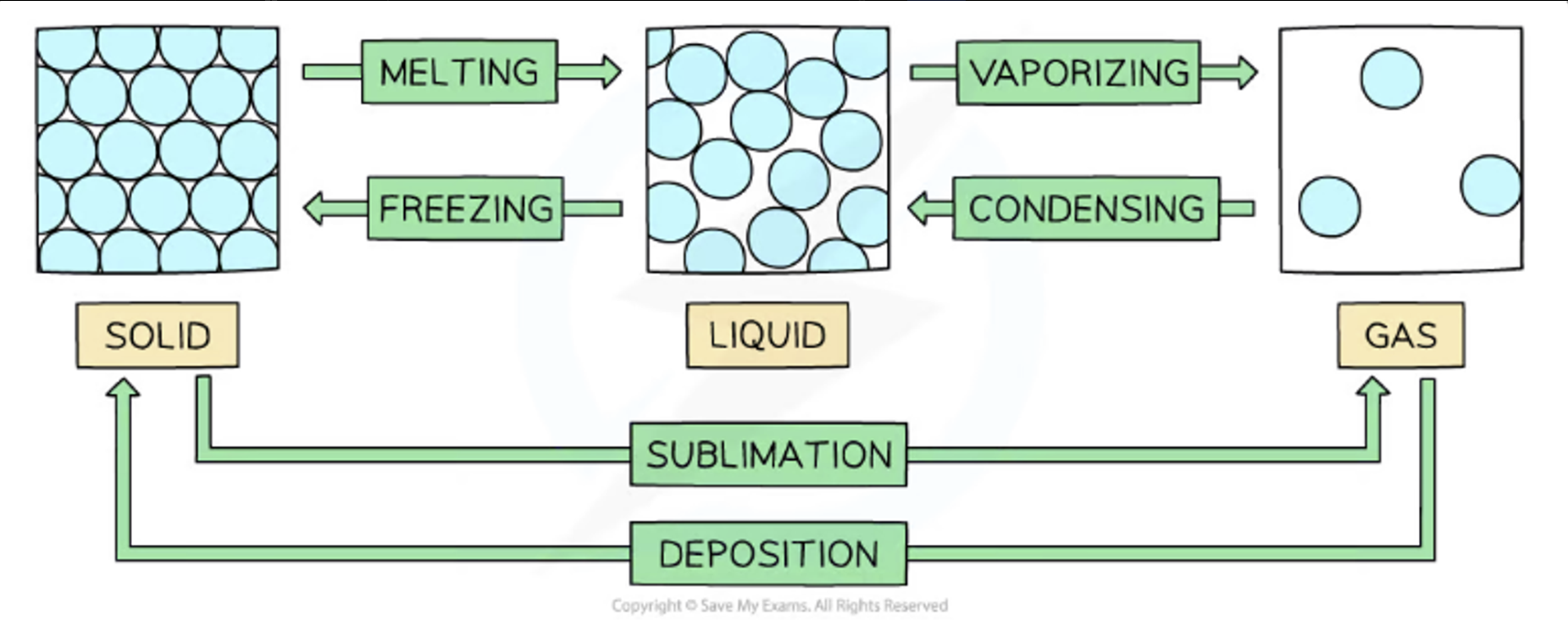
Phase Changes
Melting
Melting is when a solid changes into a liquid
The process requires heat energy which transforms into kinetic energy, allowing the particles to move
It occurs at a specific temperature known as the melting point which is unique to each pure solid
Boiling
Boiling is when a liquid changes into a gas
This requires heat which causes bubbles of gas to form below the surface of a liquid, allowing for liquid particles to escape from the surface and from within the liquid
It occurs at a specific temperature known as the boiling point which is unique to each pure liquid
Freezing
Freezing is when a liquid changes into a solid
This is the reverse of melting and occurs at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same
Water for example freezes and melts at 0 ºC
It requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature which is unique for each pure substance
Evaporation
When a liquid changes into a gas
Evaporation occurs only at the surface of liquids where high energy particles can escape from the liquids surface at low temperatures, below the boiling point of the liquid
The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
Evaporation occurs over a range of temperatures, but heating will speed up the process as particles need energy to escape from the surface
Condensation
When a gas changes into a liquid, usually on cooling
When a gas is cooled its particles lose energy and when they bump into each other, they lack energy to bounce away again, instead grouping together to form a liquid
Sublimation
When a solid changes directly into a gas
This happens to only a few solids, such as iodine or solid carbon dioxide ( dry ice )
The reverse reaction also happens and is called desublimation or deposition
 Diffusion and Dilution
Diffusion and Dilution
Definitions
Diffusion
Diffusion is the spreading out of particles in a gas or liquid. There is an over all movement of particles from areas of high concentration to low concentration.
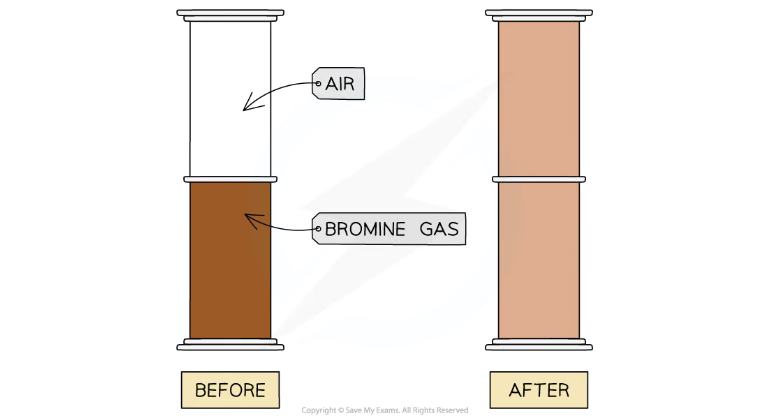
The bromine gas (Br2) becomes more pale as it diffuses into the air due to less concentration of particles.
Examples of diffusion:
Spraying perfume/deoderant
Farting

Aqueous - Dissolved in water. Written as (aq) in chemical equations.
Gaseous - Written as (g) in chemical equations.
Solid - Written as (s) in chemical equations.
Liquid - Written as (l) in chemical equations
Ammonia - NH3 - Colourless
NH3(g) + HCl(g) = NH4Cl(s)
These compounds form into the solid ring ring NH4Cl via diffusion.
The ring of ammonium chloride forms nearer to the HCl because the particles of ammonia are smaller and lighter and diffuse through the air more quickly.
Gas particles dont instantly form a solid because the may collide with air particles, and they move in random directions.
Dilution
If you pour water into orange juice, the orange colour fades. This is dilution. The more you dilute a liquid, the more the colour fades away. This is because it increases the concentration of the solute in the solvent.
It is decreasing concentration of the solute by increasing the volume of the solvent.
Solutions Terminology
Water is a universal solvent.
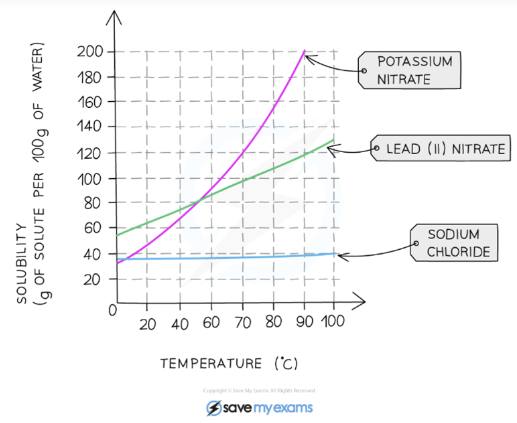
Solubility
Solubility is a measurement of how much of a substance will dissolve in a given volume of liquid.
The solubility of the solvent is dependent on pressure and temperature.
Different substances have different solubilities
Solubility can be expressed in g per 100 g of solvent
Solubility of solids is affected by temperature
As temperature increases, solids usually become more soluble
Solubility of gases is affected by temperature and pressure; in general:
As pressure increases, gases become more soluble
As temperature increases, gases become less soluble
Solubility = (Mass of solute dissolved / Mass of water)* 100
Solubility graphs or curves represent solubility in g per 100 g of water plotted against temperature
To plot a solubility curve, the maximum mass of solvent that can be dissolved in 100 g of water before a saturated solution is formed, is determined at a series of different temperatures
Elements, Compounds and Mixtures

Definitions
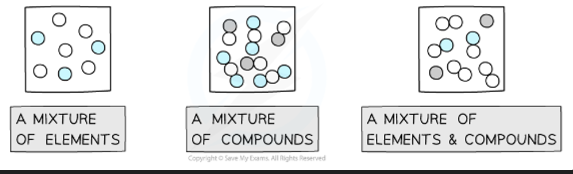
Element - Made up of only one type of atom. The simplest type of substances. - Oxygen
Compounds - A substance that contains two or more different elements chemically bonded in fixed proportions. - Water
Molecule - Two chemically bonded atoms that can be the same or different. Chemically bonded in fixed proportions
Mixture - Different substances in the same space but not chemically bonded. - Salt Water
Diatomic Molecule - An element that needs two of the same atom to be chemically stable - Oxygen
HNOF Down: Diatomic Molecules
(Go from Nitrogen to Fluorine on the periodic table then down; all of these elements are diatomic. And hydrogen!)
 Pure Substances vs Mixture
Pure Substances vs Mixture
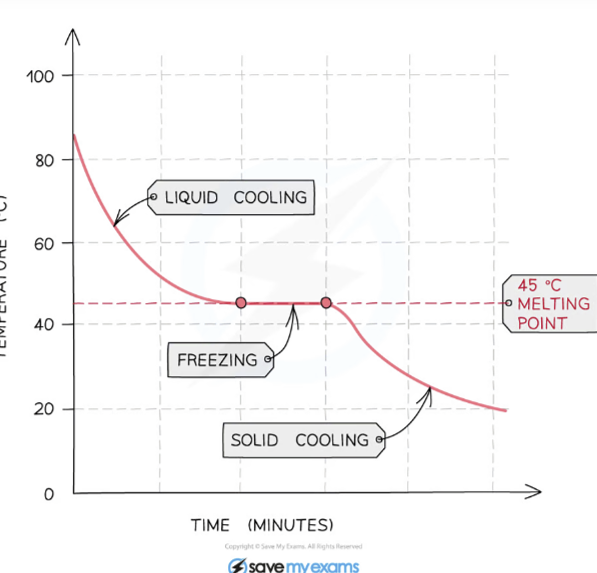
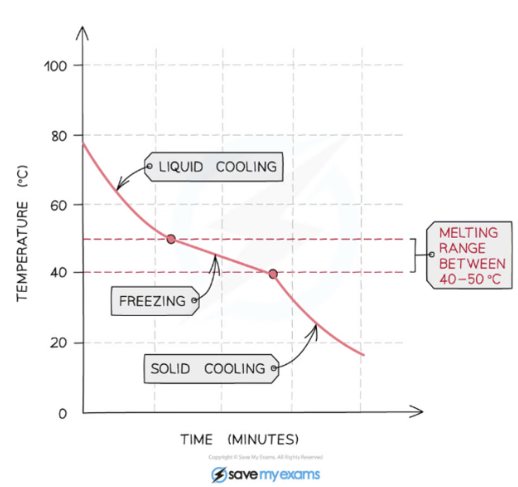
Pure substances melt/boil at a fixed temperature while mixtures melt/boil over a range of temperatures.
Foreign substances in a crystalline solid disrupt the repeating pattern of forces that holds the solid together. Therefore, a smaller amount of energy is required to melt the part of the solid surrounding the impurity
Pure Substance
Mixture (Impure Substance)
Separation Techniques
Simple Distillation
Simple distillation is used to separate soluble solute inside of mixtures while keeping the solvent.
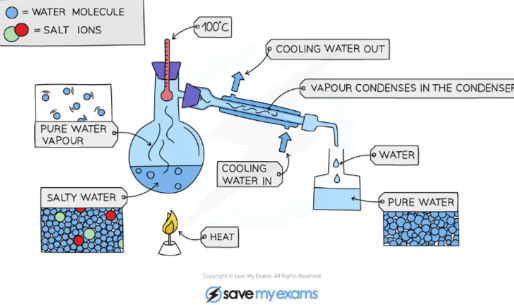
Heat the flask until the solvent in the solution evaporates.
The solvent will travel through the condenser and condense into it’s pure form, while the solute is left behind.
Water enters through the bottom of the condenser. This is because if water entered at the top of the condenser, then the water would always be leaving the condenser; never keeping it full.
Simple distillation is used when the solute and solvent have different boiling points.
Fractional Distillation
Fractional distillation is used to separate two miscible liquids.
Miscible - Solution where a liquid completely dissolves in another liquid.
The solution is heated to the temperature of the substance with the lowest boiling point.
This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker.
All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture.
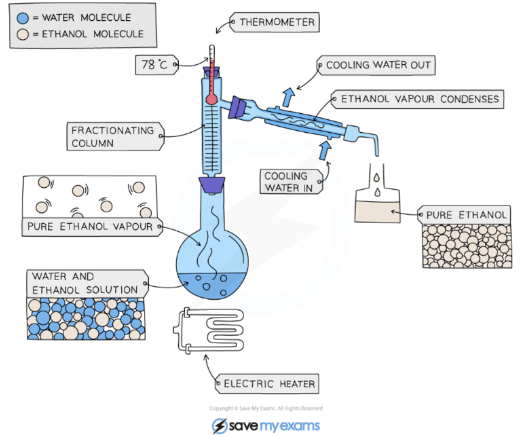
Filtration
Filtration is used when an insoluble solid is to be separated from a mixture of the solid and the solvent.
A piece of filter paper is placed in a filter funnel above a beaker
A mixture of insoluble solid and liquid is poured into the filter funnel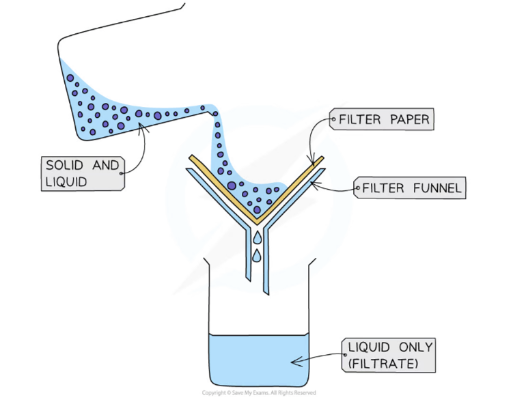
The filter paper will only allow small liquid particles to pass through as filtrate
Solid particles are too large to pass through the filter paper so will stay behind as a residue
Crystallisation
Crystallisation is used to separate a dissolved solute from a solvent while getting rid of the solvent. If you wanted to keep both the solvent and solute, you would use simple distillation.
The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
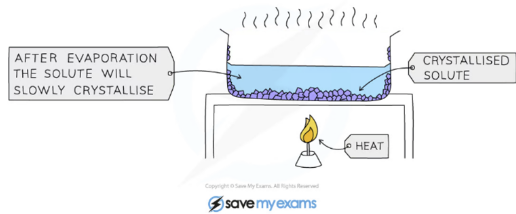 Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution
If the solution is saturated, crystals will form on the glass rod
The saturated solution is allowed to cool slowly
Crystals begin to grow as solids will come out of solution due to decreasing solubility
The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
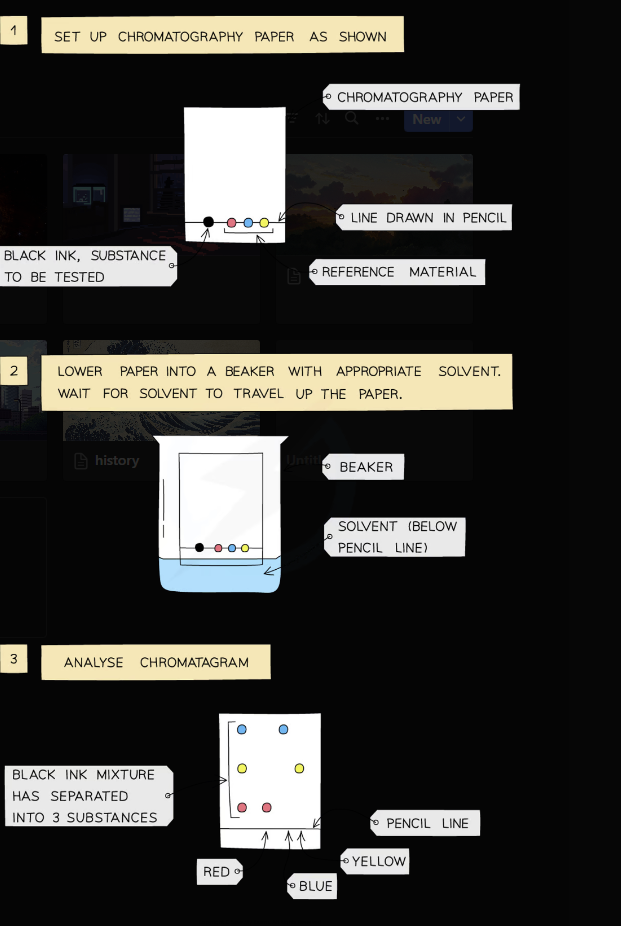
Paper Chromatography
Rf - Retention factor
This technique is used to separate a mixtures / substances that have different solubilities in a given solvent.
A pencil line is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink is soluble.
The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent, so the samples don’t wash into the solvent container
The paper is called the stationary phase
The solvent travels up the paper by capillary action, taking some of the coloured substances with it; this is called the mobile phase
Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
The substances with higher solubility will travel further than the others
This will show the different components of the mixture / substance
If two or more substances are the same, they will produce identical chromatograms
If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
An impure substance will show up with more than one spot, a pure substance should only show up with one spot
Rf = Distance travelled by solute / Distance travelled by solvent
 Atoms: Definitions and Structure
Atoms: Definitions and Structure
Atom - The simplest type of substance. Building blocks of the universe.
Molecule - Two chemically bonded atoms that can be the same or different.
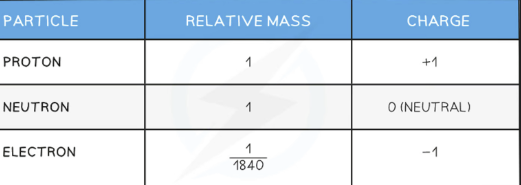
One relative atomic mass = 1/12 of a carbon12 atom.
Atoms are made up of sub-atomic particles; electrons, neutrons and protons.

Number of Neutrons = Atomic mass - Atomic number
Relative Atomic Mass - The ratio of the average mass per atom of an element to 1/12 of the mass of an atom of carbon-12.
Calculate Relative Atomic Mass

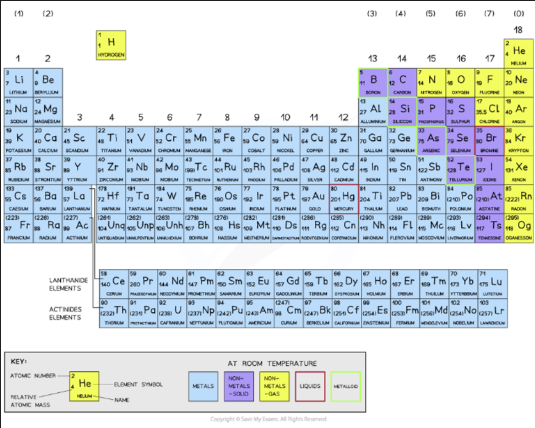
Periodic Table
Elements are arranged in increasing atomic / proton number
Groups are the amount of valence electrons each element as
Periods are the amount of electron shells there are
Electronic Configurations
Calcium, an element with 20 electrons has a configuration of 2,8,8,2
Amount of electrons in each shell
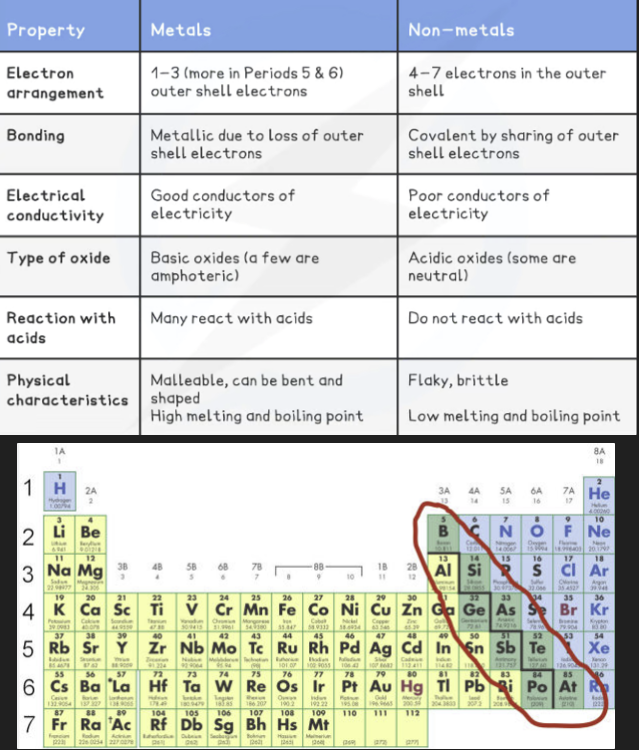
Classify Metals & Non-Metals
 Electronic Configuration & Reactivity
Electronic Configuration & Reactivity
Elements in the same group in the periodic table will have similar chemical properties
This is because they have the same number of outer electrons so will react and bond similarly
The group number of an element which is given on the periodic table indicates the number of electrons in the outer shell (valence electrons)
This rule holds true for all elements except helium; although is in group 0, it has only one shell, the first and innermost shell, which holds only 2 electrons
We can use the group number to predict how elements will react as the number of valence shell electrons in an element influences how the element reacts.
Therefore, elements in the same group react similarly
By observing the reaction of one element from a group, you can predict how the other elements in that group will react
By reacting two or more elements from the same group and observing what happens in those reactions you can make predictions about reactivity and establish trends in reactivity in that group
For example, lithium, sodium and potassium are in group 1 and can all react with elements in group 7 to form an ionic compound
The group 1 metals become more reactive as you move down the group while the group 7 elements show a decrease in reactivity moving down the group
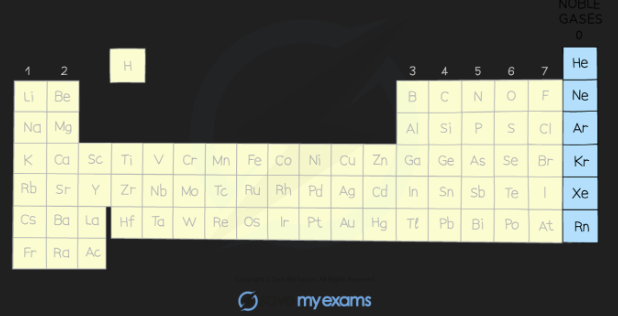
The elements in group 0 of the periodic table are called the noble gases
They are all non-metal, monatomic (exist as single atoms), colourless, non-flammable gases at room temperature
The group 0 elements all have full outer shells of electrons; this electronic configuration is extremely stable
Elements participate in reactions to complete their outer shells by losing, gaining, or sharing electrons
The Group 0 elements do not need to do this, because of their full outer shells which makes them unreactive and inert
Other than helium which has 2 electrons in its outer shell, the noble gases have eight valence electrons (which is why you may see this group labelled “group 8”)
Electronic configurations of the Noble gases:
He = 2
Ne = 2, 8
Ar = 2, 8, 8
Kr = 2, 8, 18, 8
Xe = 2, 8, 18, 18, 8
 Ionic Bonding
Ionic Bonding
Ion - An atom with a positive or negative charge. The atom loses or gains an electron to have a full outermost shell.
Anion - Negatively charged ion
Cation - Positively charged ion
All metals lose electrons and form cations. ( Except transition metals )
All non-metals gain electrons and form anions.

Diagram showing the formation of the sodium ion

Diagram showing the formation of the chloride ion
Common Ions
Ag+= Silver
NH4+= Ammonium
H+ = Hydrogen
OH- = Hydroxide
NO3- = Nitrate
Fe2+ = Iron(II)
Pb2+ = Lead
Zn2+ = Zinc
Cu2+ = Copper
SO4 2- = Sulfate
CO3 2- = Carbonate
Fe 3+ = Iron(III)
PO4 3- = Phosphate
Facts
Ionic compounds are normally solid at room temperature and are non-volatile (doesn’t vaporise easily).
They are usually water soluble because ionic compounds and water are polar substances.
Ionic compounds can conduct electricity when molten. The giant lattice structure is broken and ions are free to move around and carry charge.
Covalent Bonding
Covalent Bonding - The electrostatic forces of attractions between the oppositely charged positive nuclei and shared pair of electrons.
Simple Molecular Structure - Covalent bonds joining the non-metal atoms together but intermolecular bonds that act between neighboring molecules. This makes the structure weaker than a giant covalent structure.
Giant covalent structure - Covalent bonds joining all non-metal atoms together. High boiling and melting point
Viscosity - How thick a liquid is
Broken intermolecular forces are a physical change
Broken covalent bonds are a chemical change
The higher the relative molecular mass, the higher the boiling / melting point of a molecule
Covalent structures do not conduct electricity.
Only graphite does.
Diamond, graphite and sand are giant covalent structures. Diamond is composed of only carbon and each carbon atom has 4 covalent bonds. Sand is silicon dioxide. Each silicon atom is bonded to three oxygen atoms.
Graphite is made of layers of carbon atoms. To connect these layers there are intermolecular forces.
Each carbon atom has 3 covalent bonds, leaving one delocalised electron. It is able to move freely and carry charge, and is why graphite can conduct electricity.
Graphite is soft as the layers can slide over each other.
Metallic Bonding
Metallic Bonding - The electrostatic forces between cations and delocalised electrons.
Metals are good conductors of heat and electricity. This is because the delocalised electrons move freely and can store electrical currents and thermal energy
Alloys are mixtures of metals, where the metals are mixed together physically but are not chemically combined
They can also be made from metals mixed with nonmetals such as carbon
Alloys often have properties that can be very different to the metals they contain, for example they can have greater strength, hardness or resistance to corrosion or extreme temperatures
Alloys contain atoms of different sizes, which distorts the regular arrangements of atoms
This makes it more difficult for the layers to slide over each other, so they are usually much harder than the pure metal
 Knowt
Knowt