Ionic Bonding
Definition: electrons being transferred between atoms specifically between the non metal and metal where the metal is always the electron donor and the non metal is the electron acceptor
they attain their nearest inert noble gas configuration
atoms with greater electronegativity difference
the higher energy electrons leave the less electronegative element
all ionic compounds dissolve in water
forms crystalline structure
Chemical Formulas
*like a reduced fraction, instead of saying 6/8 you can say ¾
Ionic compound: an array of cations and anions (can vary from sample from sample)
formed from a cation and an anion (negatively charged ion)
have electrically neutral formulas ( same number of gain and loss of electrons )
subscripts tells to balance the charges to get the electrically neutral compound
Monoatomic: one atom in ions
Polyatomic: many atom ions (charged groups of covalently bound atoms)
https://www.studystack.com/flashcard-907510
Main group ions: First two and last six group
Oxidation States, Polyatomic ions
Ion formtaion:
Metals
small ionization energy lose electrons and become cations
Non Metals
large electronegativity
low energy open orbitals
gain electrons often by taking the electrons away from metals forming anions
Driving forces:
system wants to lower its energy by leaving metals and joining metals
atoms of the main group want to become isoelectronic with the noble gas
Oxidation States: electron accounting system (treat most bonds as ionic bonds)
follow in order
1) Pure elements have an oxidation state of 0
2) A compound with fluorine and a different type of element: Fluroine has an oxidation sttae of 1-
3) Metals: 1A are +1, 2A are +2 and AL are +3
a) alkali earth metals
a) 1A: Li, NA, K, Rb
2A: Be, Mg, Ca
4) H is usually +1 (which is a proton) (can be -1 with a metal if it encounters the metal like sodium)
because hydrogen has an open orbital inner energy H^-1 = hydride w two electrons
5) O is usually -2 but it can be -1
6) Halogens are usually -1
oxidation states should result in a net charge of 0
Ions: overallcharge on ion = sum
Neutral molecules: sum = zero
F2 = molecule in elemental state
oxidation state=0
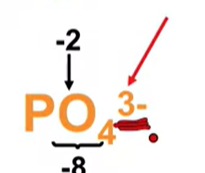
needs to add up to -3 since it’s the overall charge
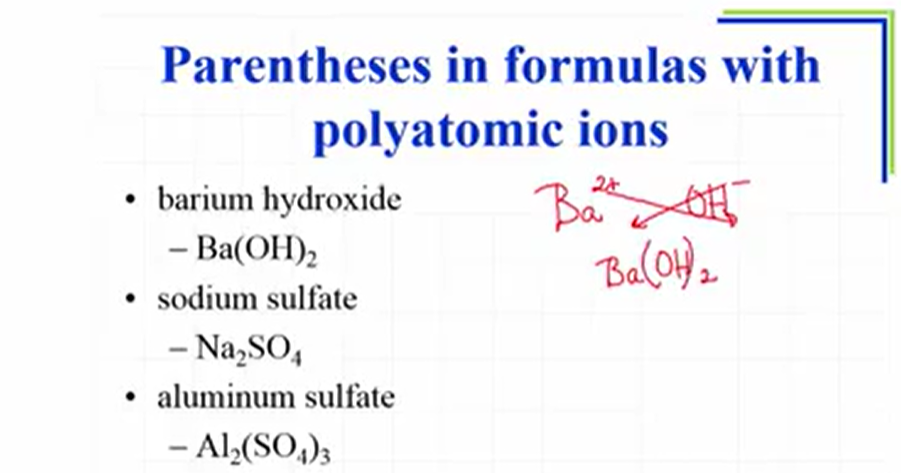
Naming ionic compounds
Naming Ions (combination of cation + anion)
monoatomic cation: no change in name
monoatomic anion: change the ending of the name of the element to -ide
fluorine F1- fluoride
2 cases for compounds
Cation can only assume a single oxidation state - true for alkaline earth metals, a few of transition metals and aluminium
sodium +1 charge sodium ion or sodium anion
alkaline earth metals: calcium (metallic calcium calcium 0) or lose its valence electron for charge +2
transition metal: silver has plus one oxidation state, zinc +2, aluminum +3
Name: cation anion (ide)
Some instances where there’s groups of non metals that make a cation (ammonium) & still have an ionic compound
second case (when metals adopt more than one oxidation state (most transition metals and larger Group 3A and 4A metals)
oxidation states indicated in roman numerals in parantheses after the metal
applies to transition metals (3-12)
exceptions: silver and zinc
Name: metal (oxidation state) anion
Examples: FeBr2 is iron(II) bromide

More electrons in the same level, gets closer Cl is smaller than NA.
Particle diagram of Hallite and Mica:
