CHEMISTRY II
Characteristics of Solids and Liquids
Solids, which can be classified as:
Amorphous - No arranged structure of molecules
Crystalline - Molecules arranged into patterns
Liquids, which have special properties, such as:
Surface Tension
Viscosity
Vapor Pressure
Boiling Point
Heat of Vaporization
Intermolecular Forces of Attraction
Dipole-Dipole — Created between polar molecules, always partially positive and partially negative.

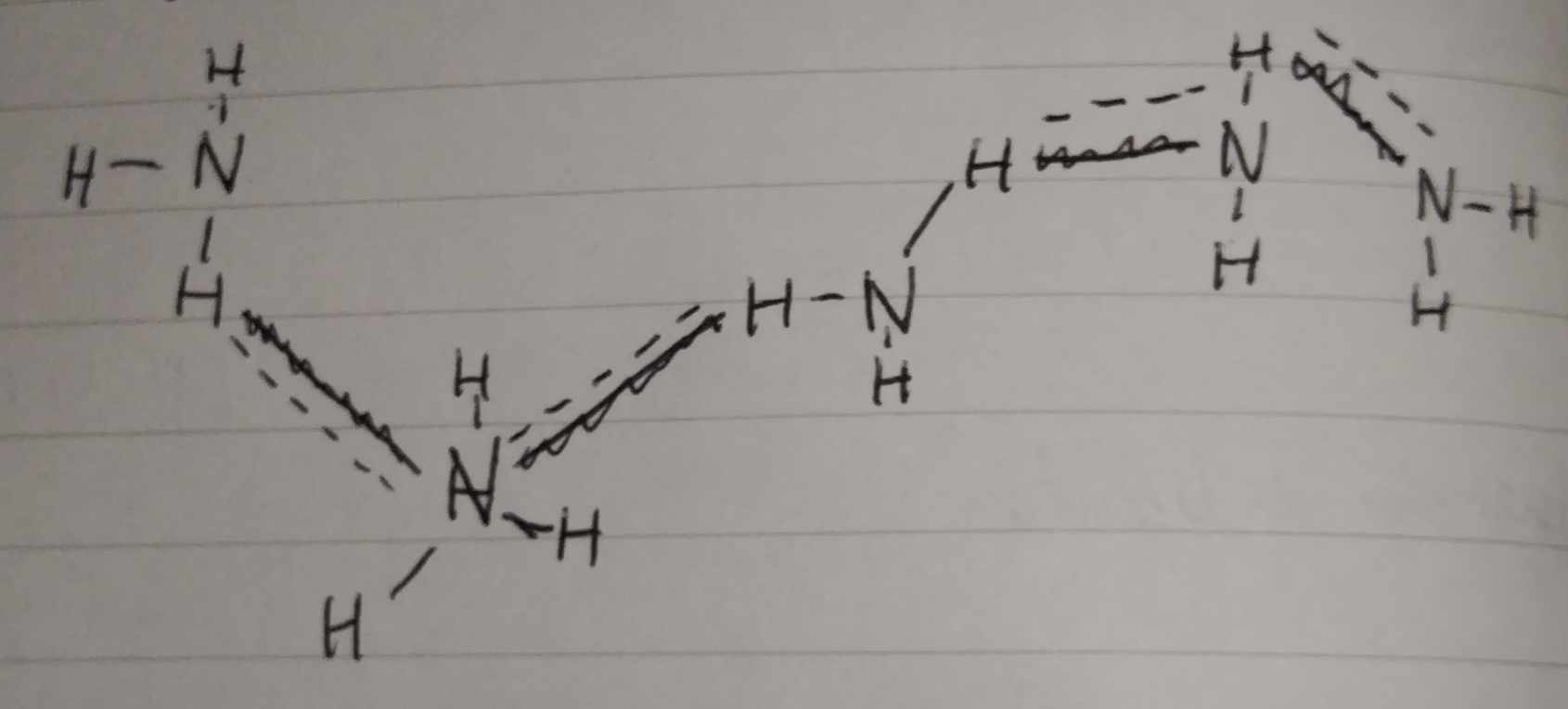
Hydrogen Bond — A special type of dipole-dipole (hydrogen). It is 5x to 10x stronger than dipole-dipole. It is intermolecular in nature. It always bonds to Nitrogen, Oxygen, or Fluoride.
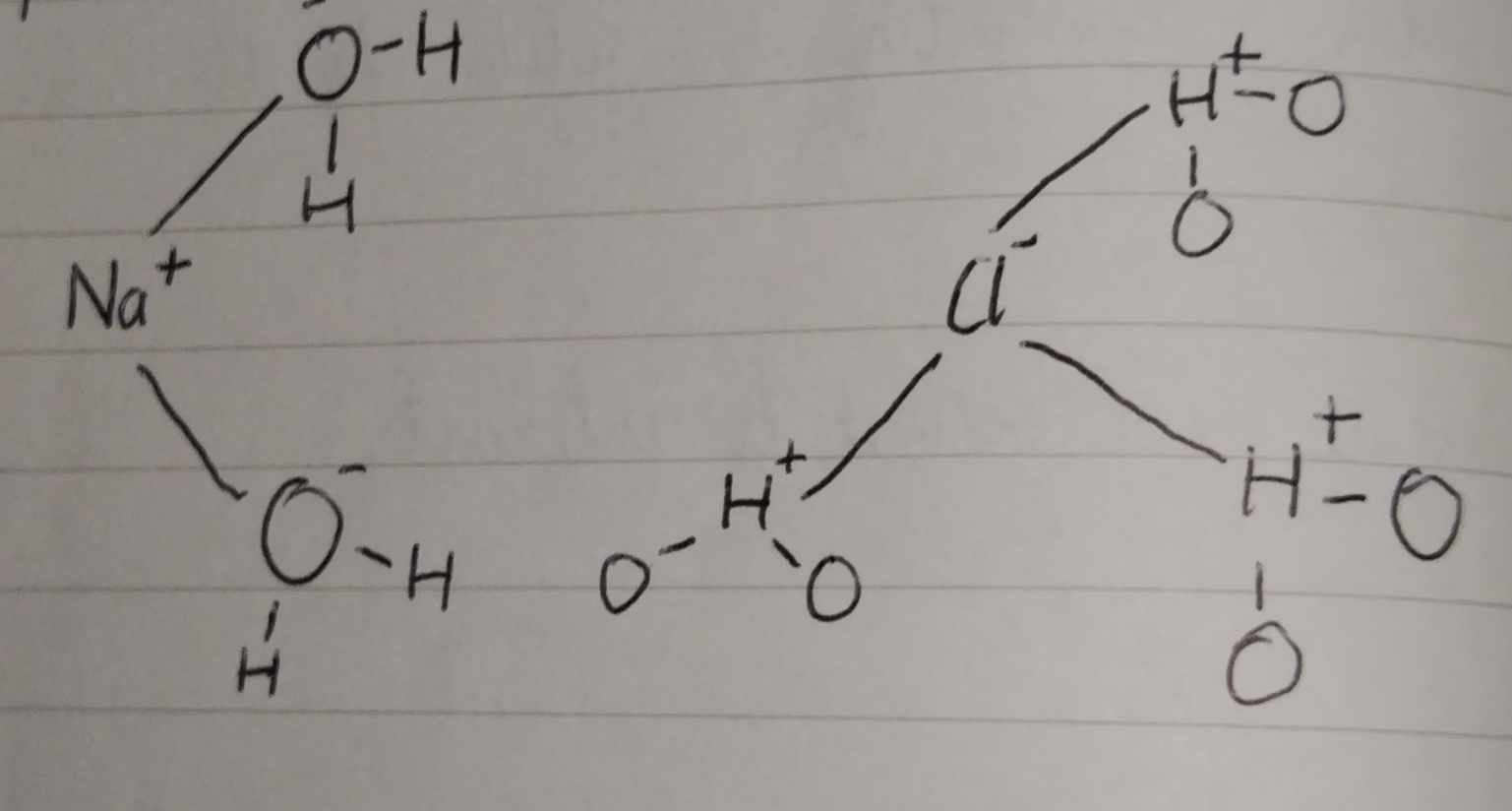
Ion-Dipole — Acts between an ion(+,-) and a polar molecule
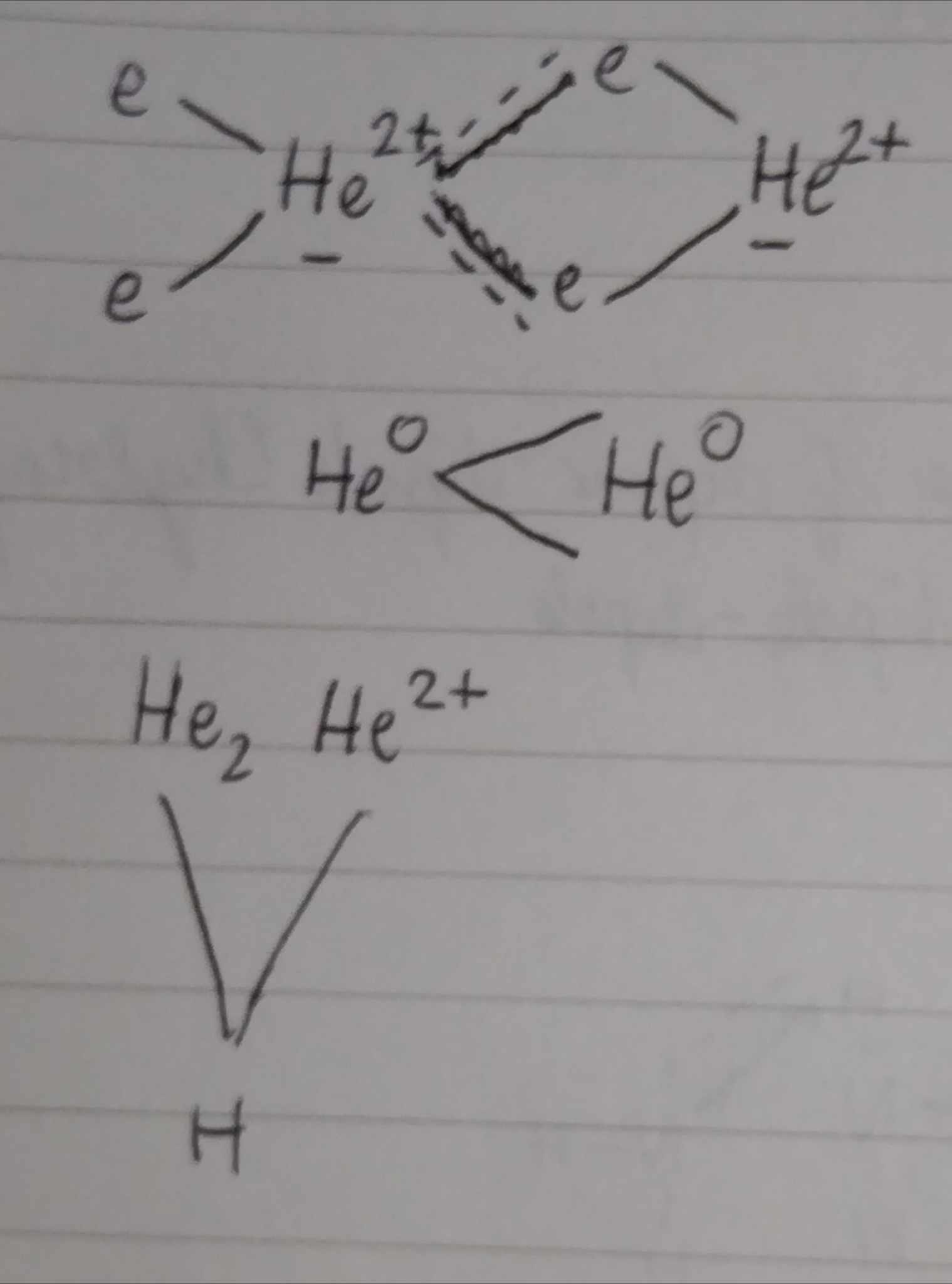
London Dispersion Force — Between all atoms and molecules, Non polar (zero dipole moment)
Note: - - - - - - = Bond ————— = Connection
3 steps in the formation of liquids
“Expanding the solvent” - Overcoming the intermolecular forces in the solvent to give room for solute.
“Expanding the solute” - Separating the solute into it’s individual components/
Allowing the solute and solvent to interact.
Enthalpy of Solution
HSolution = H1+H2+H3
(+) - Endothermic (Heat Absorbed)
(-) - Exothermic (Heat Radiated)
Concentration
Percent by mass and volume and Mass-Volume
% by mass = (mass of solute(g) / mass of solution(mL)) × 100%
% by volume - (volume of solute(g)/volume of solution(mL))
% by mass-volume = (mass of solute (g) / volume of solution (mL)) × 100%
Mass of solute (m) = Molarity (M) × Volume (L) × Molar mass (g/mol)
Solution Stoichiometry
Molarity - # of moles of solute dissolved in a solution
Molarity (M) = moles of solute / liters of solution
Molality - # of moles of solute dissolved in 1 kg solution
Molality (m) = moles of solute / mass of solvent (kg)
1st Law of Thermodynamics
Law of Conservation of Energy - Energy cannot be created nor destroyed, only transformed.
Physics: U = Q - W; Work is done by the system
Chemistry: U = Q + W; Work is done on the system
Note: W = (-) done by the system
W = (+) done on the system
Q = + (absorbs Heat) Endothermic
Q = - (loses Heat) Exothermic
3 types of system
Open - Matter and Energy freely move from system to surroundings and vice versa. (Example: Open teakettle)
Closed - Energy freely moves from system to surroundings and vice versa. While Matter is restricted from moving. (Example: Closed teakettle)
Isolated - Energy nor matter cannot freely move from system to surroundings and vice versa. (Example: Vacuum Chamber)
3 types of heat transfer:
Conduction - Heat transferred through contact (solids)
Convection - Heat transferred through fluid movement (gas or liquid)
Radiation - Heat transferred through electromagnetic waves
Diatomic Elements
Nitrogen (7) - N2-3 (14.01 g)
Oxygen (8) - O2-2 (16.00 g)
Fluorine (9) - F2- (19.00 g)
Chlorine (17) - Cl2- (35.45 g)
Bromine (35) - Br2- (79.90 g)
Iodine (53) - I2- (126.90 g)
Hydrogen (1) - H2+ (1.01 g)
Halogen Group
Fluorine (9) - F2- (19.00 g)
Chlorine (17) - Cl2- (35.45 g)
Bromine (35) - Br2- (79.90 g)
Iodine (53) - I2- (126.90 g)
Astatine (85) - At- (210 g)
Tennessine (117) - Te- (294 g)
Important Elements / Compounds
Sulfate - SO4-2 (96.06 g/mol)
Ammonium - NH4+ (18.04 g/mol)
Aluminum - Al+3 (26.98 g/mol)
Phosphorus - P-3 (32.06 g/mol)
Carbonate - CO32 (60.01 g/mol)
Nitrate - NO3- (62.01 g/mol)
Chlorine - Cl2- (35.45 g/mol)
Hydroxide - OH- (17.01 g/mol)
Hydrochloric Acid - HCl-1 (36.46 g/mol)
Sulfuric Acid - H2SO4+6 (98.08 g/mol)
Carbon - C+4 (12.01 g/mol)
Properties of Periodic Table Groups
Alkali Metals (GROUP 1/1A)
Less dense than other metals
One loosely bound valence electron
Highly reactive, with reactivity increasing moving down the group
The largest atomic radius of elements in their period
Low ionization energy
Low electronegativity
Alkaline Earth Metals (GROUP 2/2A)
Two electrons in the valence shell
Readily form divalent cations
Low electron affinity
Low electronegativity
Transition Metals (GROUP 3-12/1-10B)
Lanthanides (rare earth) and actinides are also transition metals. The basic metals are similar to transition metals but tend to be softer and hint at nonmetallic properties. In their pure state, all these elements tend to have a shiny, metallic appearance. While there are radioisotopes of other elements, all actinides are radioactive.
Very hard, usually shiny, ductile, and malleable
High melting and boiling points
High thermal and electrical conductivity
Form cations (positive oxidation states)
Tend to exhibit more than one oxidation state
Low ionization energy
Metalloids or Semimetals
Electronegativity and ionization energy intermediate between that of metals and nonmetals
May possess a metallic luster
Variable density, hardness, conductivity, and other properties
Often make good semiconductors
Reactivity depends on the nature of other elements in the reaction
Nonmetals
Halogens and noble gases are nonmetals, although they have their groups, too.
High ionization energy
High electronegativity
Poor electrical and thermal conductors
Form brittle solids
Little if any metallic luster
Readily gain electrons
Halogens
The halogens exhibit different physical properties from each other but do share chemical properties.
Extremely high electronegativity
Very reactive
Seven valence electrons, so elements from this group typically exhibit a -1 oxidation state
Noble Gases
The noble gases have complete valence electron shells, so they act differently. Unlike other groups, noble gases are unreactive and have very low electronegativity or electron affinity.