Soil science exam #2
Soils + the hydrologic cycle
Water balance
SS = P - ET - D
Evapotranspiration = evaporation + transpiration
Transpiration - loss of water from plants such as trees + grasses
Evaporation : loss of water from water bodies and land surface
Water balance over a year
Precipitation > ET surplus can go to soil storage groundwater recharge runoff
ET> Precipitation Drawing down stores soil water
Water balance over a year in an arid environment
This is because the ET is pushing up against precipitation, no opportunity for excess precipitation
Potential evapotranspiration
Amount of water respired from a well watered, densely vegetated system
Actual ET may not reach PET - because systems are not always well watered
Estimation techniques often incorporate multiple components of ecosystems (radiation, temp, wind, and atmospheric pressure)
Soil aeration
The process by which air in the soil is replaced by air in the atmosphere
A well aerated soil the soil air is similar in composition to atmosphere above and poorly aerated contain more CO2 and less O2
Soil aration in terms of gaseous composition of soil air
Soil aeration primarily controlled by
Soil water content
Soil mnacroporosity
Rate of O2 consumption in the soil
Influence of water on soil aeration
Soil water reduces soil aration
Because rate of O2 diffusion through water is many times slower than the rate of O2 diffusion through air
Water filled pore space
% water filled pore space = volume of soil water / volume of soil pores
Soil is saturated when water filled pore space = 100%
R
Rate of O2 consumption of soils
Micorbial activity involves microbial respiration which consumes O2 and produces CO2
Microbial activity increases with temp, up to a maximum
Therefore, rate of O2 consumption increases with temp
Aerobic soil = has oxygen
Anaerobic soil = lacks oxygen
Wetland soils
Wetland soils are water saturated for prolonged periods, when soil temperatures and other conditions are such that plants and microbes can grow and remove oxygen, thereby assuring anaerobic conditions
Wetlands have hydric soils
There are often Histosols
PLant adaptation to waterlogged environments aerenchyma
Aerenchyma: large intracellular structures (pore spaces) which extend through the entire plant and allow for storage and transport if gas to submerged roots
CO2 in soil air
soil CO2 concentrations are
A. Higher in June than in November
B. Lower in June than in November
C. The same between June and November Range
Rasnge of possible soil temp and their implications for soil processes
Daily variations in air temp near earth's surface is controlled mainly by the input of energy from the sun (yellow) and output of energy form the surface (blue)
Soil temp - dinural cycles
Huge range near surface
At 0.5 cm, > 20 C range
Max. temperature ~ 2pm
Dampened and delayed with depth
At 10 cm, < 6 C range
Max. temperature ~ 6pm
Negligible diurnal cycle at 80cm
Soil temp - seasonal cycles
Soil temps fluctuate more at surface than at depth
2/19./24
ALbedo - proportion of radiation that is reflected by a surface
High albedo, low albedo
Bare soil generally has a lower albedo (absorbs more heat than a soil with crop residue
Water will be cooler due to specific heat capacity
Specifc heat capacity: the amount of energy required to raise the temp of a unit of a substance by 1 degree C
Specific heat capacity of water and sand are different
A dry soil warms up easier than wet soil
Due to higher specific heat capacity of water compared to soil minerals
Soil temp in different tillage systems
2/22/24
Soil colliods - Organic and inorganic matter with very small particle size and a correspondingly large surface area per unit of mass
Sand silt clay are sometimes called soil separates
Soil texture is sometimes called soil particle size distribution
Soils with fine particles have a greater soil surface area than soils with coarser particles
Crystalline
Denotes a definite chemical compositions with planner surfaces and regular angles
Atomic arrangement of quartz shows planar surfaces and regular angles
However crystalline silicate clays in soil are not formed from disintegration of large crystals into smaller crystals
Crystalline silicare clays are among new minerals - pedogenic clay s
SOil colloids, including crystalline silicate clays contribute enormously to soils cation exchange capacity (CEC)
In cation exchange, cations absorbed to negatively charged clays exchange with cations in soil solution
Basic structural components of silicate clay
Tetrahedral and octahedral sheets stack in different configurations in the different types of crystalline silicate clays
1:1 tetra to octa hedral
A loose metaphor 1:1 clays are a stack of break and peanutbutter, low filling diversity
2:1 tetra to octa
2:1 clays are a sandwich with lots of fillings
Different layer structures of silicate clays
Biotite and muscovite micas are primary minerals, here we discuss secondary minerals also called micas
Crystalline silicate clays in context
Kaolinite - white gold - in industrial uses
Minimal shrink, preferred fr ceramics
Iron impurities make it red
Expanding 2:1 clays - smectite
Gives vertisols their shrink- swell
Have industrial applications
Where swelling when wet is needed to create seal
NON crystalline silicate clays
Allophane is a non crystalline silicate clay composed of Si, Al and O atoms, not arranged in crystalline sheets
Primary constituent of volcanic soils (andisols)
Processes leading to soil charge
Constant charge due to isomorphic substitution
The process of replacing one structural cation for another of similar size
Net charge difference is -2 from a Si +4 to Mg2+
Often in soil cations are replaced with less positive cation, leading to net negative charge
pH dependent change
2/26/25
Soil colloids 2
Review from last cass, what makes a soil colloid a soil colloid
Organic and inorganic matter with very small particle size and a correspondingly large surface area per unit of mass
WHat is a crystalline silicate clay
Denotes a definite chemical compositions with planner surfaces and regular angles
Atomic arrangement of quartz shows planar surfaces and regular angles
However crystalline silicate clays in soil are not formed from disintegration of large crystals into smaller crystals
What features distinguish the types of crystalline silicate clay
Iron and alluminum oxides
Here showing Gibbsite
An aluminum oxide clay common in highly weathered soils
Octahedral sheets hydrogen bonded together
Other oxide- type clays can have iron instead of aluminum, or be less crystalline structure
Organic colloids
Also known as soil organic matter
Non crystalline structure
-OH hydroxl groups
What happened to the dyes - yellow is more negative and bleu is more positive so blue sticks to the soil!!!!
Processes leading to soil charge
Constant charge - due to isomorphic substitution
pH-dependent charge
“Such as substitution [isomoprhic; reduction in charge ] commonly occurs in aluminum dominated dioctahedral sheets.”t
Another view of isomorphic substitution
Process through which structural cation and shapes are exchanged with similar cation that leads to a net negative charge
Processes leading to soil charge
pH-dependent charge
Hydroxl (-OH) functional groups exist on the edged of inorganic colloids and organic colloids alike
We can consider PH dependent charge by imagining the hydroxl group on the edges of clays and on organic compounds as a 2 car garage
House is the oxygen atom
Cars are hydrogen ions
PH as a reflection of hydrogen ion concentration in a substance
Decrease in soil ph is like a football game happening in the neighborehood
WHen there is 2 the charge becomes +1
Increase in soil ph is everyone is gone, charge becomes -1
More acidic - low ph - positive charge - greater anion exchange capacity
More alkaline - high ph - more negative - greater ion exchange capacity
When we sum exchange sites in soil, we report units of charge per mass (soil or colloid)
For CEC, the sum is of negative charges
PH- dependent negative charge increases as soil pH increases although degree varies with soil colloid
As a function of soil ph
Key point
Weathering of clays follows a general trend in which:
2:1 clays weather into
1:1 clYS WHICH WEATHER into
Fe and Al oxides
Driven by leeching of silica and cations
Constant charge of soil colloid decreases with weathering →
Princinples of CEC that contribut to low CEC in southeastern US
Highly weathered clays developed under warm humic climate
ALso
Lower organic matter high decomposition rates
More acidic soils → ph dependent charge leads to anion exchange capacity
4 rules gocerning cation exchange
1. Reversabilty
WHat goes on may come off
2. Charge equivalence
One +1 cation for another +1 cation or two +1 cations or one +2 cation etc
3. Ratio Law
The ratio of two different cations in soil solution will equilibriate with those absorbed to exchange complex
4. Cation selectivity
SOme cations are held more tightly on exchange complex than other →
The view of cations floating in a solution by themselves is a simplification because cations are usually hydrated
hydrated radius describes the effective size of cation in solution
Measuring cation exchange capacity
Additon of NH 4 to soil
Replaces other action son the exchange matric these cations are leached into beaker and excess NH is removed with organic solvent
Very high concentration K+ solution is used to replace and leach absorbed NH4
NH4 and K+ have similar hydrated radii so ratio law comes into effect
Amount of NH4 leached from osil can then be quantified representing total negative charges )CEC) fron soil
WHy do we use NH 4 to measure cation exchange
Small hydrated radius makes it more likely to:
Replace larger more hydrated cations
Not be displaces by larger more hydrated cations
NH4 in solution can be easily measured
2/28/24
Mesuring cation exchange
Additon of NH 4 to soil
Replaces other action son the exchange matric these cations are leached into beaker and excess NH is removed with organic solvent
Very high concentration K+ solution is used to replace and leach absorbed NH4
NH4 and K+ have similar hydrated radii so ratio law comes into effect
Amount of NH4 leached from osil can then be quantified representing total negative charges )CEC) fron soil
WHy do we use NH 4 to measure cation exchange
Small hydrated radius makes it more likely to:
Replace larger more hydrated cations
Not be displaces by larger more hydrated cations
NH4 in solution can be easily measured
Soil organic carbon increases soil CECm and does this to greater extent in high pH soils
Soild higher in CEC - whether due to organic colloids, inorganic colloids, or both- have greater capacity to prevent nutrients cations from leaching
SOIL ACIDITY
Features of a log scale
1 each gardation of “1” on a pH scale represents a 10 fold difference in H+ ion concentrations
2. Absolute change in H+ iron concentration for 1 unit pH change is much greater on the acidic side than on alkaline side of scale
PH range of soils and other materials
To understand how acidity develops over time, where does it come from?
Sources of soil H+ in soil
Dissociation of carbonic acid from CO2
Process if molecules splitting apart
High concentrations of CO2 in soil are dissolve into soil solution which then forms carbonic acid, when then dissociates to bicarbonate and H+
DIssociation of acidic functional groups on organic matter (from plants)
Functional groups = specific groups of atoms within molecules that have their own characteristic properties regardless of the other atoms present in a molecule
Oxidation of ammonium (NH4) to nitrate (NO3-) releases two H+
Microbes oxidize NH4 as an energy source through process known as nitrification
This contributes to acidification of ammonium based fertilizers
Oxidation of sulfer
Either through organic matter that contains SH groups
Or through pyric oxidation FeS2
Input of acids in precipitation
Sulfur dioxide (SO2) and Nitrogen oxifes (NO) are released from fossil fuel combustion
Undergo atmospheric reaction to form acid rain
When dissolved in rainwater and dissociate
Generating acidity
Plant roots taking up cations then releasing H+ to maintain their charge balance
Plant roots cells need to maintain a charge balance across their cell membranes
Therefore if one positive charge (nutrient cation) goes in, one positive charge (H+ or other cation must go out
Plant roots taking up anions, then releasing bicarbonate HCO3 to maintain charge balance
Reduction of nitrate to nitrogen gas ( denitrification)
Types of soil acidity
Active: in solution
Exchangeable: held ner colloid surfaces
Residual: tightly bound to colloid surfaces
Active acidity is a very small amount of acidity compared to exchangeable and residual acidity.
Acid cations = cations that generate and H+ aqueous solution in soil, thes are H+ and Al3+
AL3+ generates H+ by hydrolyzing water and combining with resulting OH 0
One AL3+ can erelase up to three H+ ions
Soil pH will decrease but to a lesser extent of that of water if you ad 3cMol of acid to soil on pH
Because soil has a buffering capacity
Buffering: An addition of acidity will cause more acidity to move to exchangeable acidity in soil colloids so the addition of acidity is not fully reflected in active measured acidity
Mechanisms of pH buffering
Protonation and deprotonation of organic matter functional groups (R-OH)
Gaining or giving protons H+
Protonation and deprotonation of pH- dependent charge sites
Cation exchange reactions
Reactions of aluminum and carbonates
Soils become acidic when
H+ ions are added to soil
Thes H+ solutions exchange with nonacid cations Ca2_ Mg 2+, K+ Na+ held on colloids
Noncaid cations are leached way (bc they travel with anions)
In arid regions nonacid cations remain in soil and re-exchange with acid cations, preventing a drop in pH level
An acidic soil has an exchange complex dominated by acid cations
WHat about this soil propoerties might be different
Organic matter of inorganic colloids could influence buffering capacity which is why they are different, orange is sandier
L
L
Acidic organic material
High rainfall
Parent material low in nonacidc cations
Sandy soiuls (low buffering capacity)
3/3/25
Hydration of cations influences their effective radii, and therefore how easily they are replaced in a cation exchange
Larger hydrated cations have weaker bonds and therefore are replaced easily
Sources of H+
Respiration- dissociation of carbonic acid
Decomposition of organic matter
Oxidation of ammonium based fertilizers
Acid saturation
Recall percent base saturation
We calculate acid saturation using same approach
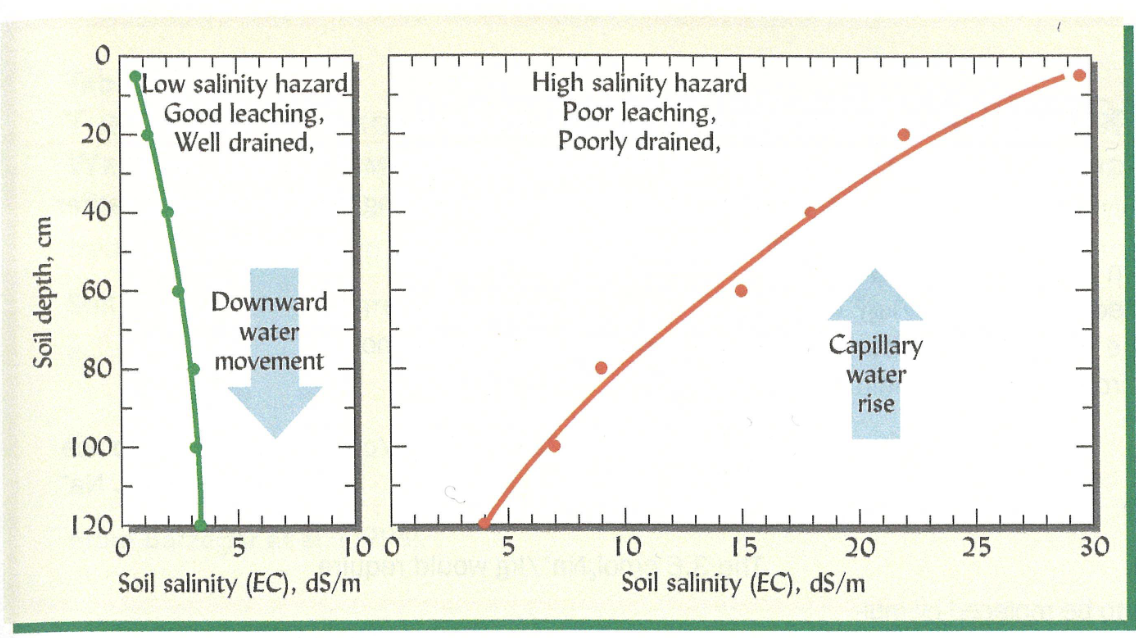
Acidity throughout the soil profile
Given The sources of H+, which pH graph would you predict is more likely found in a humid climate
Surface of soil is more subject to plant matter composition and weathering
More acidic in the higher soil and less acidic in the lower soil
Solubility of aluminum declines rapidly at soil pH above ~5.0-5.5
Inputs of acidic organic material mobilize AL3+
Fewer H+ sources; Al precipitates, contributing to formation of Bs horizon
Soil pH and crops
Some crops prefer acidic soils, some prefer neutral, some prefer alkaline soils
Justus von liebig's law of the minimum published in 1873
“If one growth factor/nutrient is deficient. Plant growth is limited, even if all other vital factors / nutrients are adequate…. Plant growth is improved by increasing the supply of the deficient factor /nutrient “
Modified truog diagram which purports to show nutrient availability across the range of soil pH:
Limitations:Width of band is not actual amount of nutrient
Even at widest part of band, nutrient may not be non limiting for plant
Even at narrowest part of band nutrient may not limit plant growth
Diagram implies that optimal soil pH is about 6.5, but crops can be highly productive outside this range
Even if topsoil pH is low, low ca, plants may uptake Ca from subsoils
Limitations; more recent
Plant roots and soil particles both have pH dependent charges and nutrient availability is mediated by both plant and soil charge
Evidence of plant uptake and colloid resorption following apparently opposite patterns
pH conditions with most absorption of colloid are same as pH conditions that make it best for plant uptake
Many unknown remain regarding role of pH in nutrient availability
Contrary to statement that remain popular in agronomic texts the soil pH cannot be used to predict or estimate plant nutrient availability
What is well established regarding mechanisms of crop preference for soil pH labels
Nutrient mineralization increases with pJ
More in N cycling later classes
Aluminum toxicity at low pH
Aluminum toxicity at low pH
At pH <5.5 aluminum is in the Al3+ form and competes with the essential nutrients like Ca 2+ Mg2+_ and K+ for negatively charged exchange sites
Plants can experience toxicity form taking up Al3+ and trying to use it in palace of Ca2+
Aluminum takes hydrogen and generates hydrogen irons and lowers pH
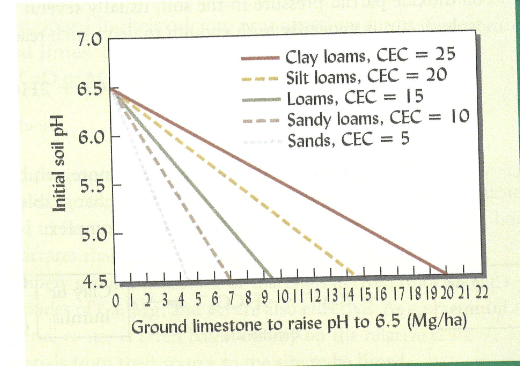
Why do we lime soils
We lime soils because it helps us to neutralize soil acidity and increase soil pH
Acid cations in lime can replace cations in solution of soil
The greater the buffering capacity of soil the more lime is needed to realize the pH
Effect of limiting in raising pH is greatest in horizon is application
Evenbut dilute increase in pH
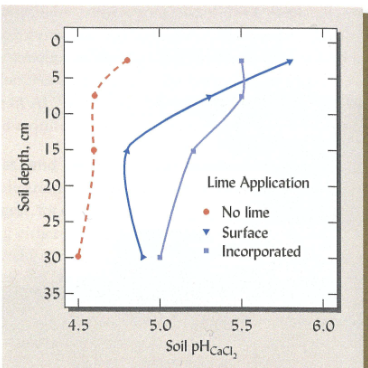
Liming generally needs to be repeated over time
because water and effects can change the liming effects
Alkaline = pH above 7 = more OH- ions
Alkaline soils are mostly found in arid reagions
Arid regions have limited sources of H+ due ot low biological activity
Arid regions experience limited leaching of Ca2+, Mg 2+ K+ and Na+
Features of soils in arid regions
Water limitation
Potential evapotranspiration > precipitation
PET - potential could be greater than what is actually evaporating
In arid environments theres a larger demand for water in environment
demand for water is greater than water that is going into soil s
Island of fertility
Plants protect soil from erosion and promote water infiltration and storage
Grazing animals concentrate manure to grazed areas providing more organic matter
leading to fertility to suppport more plant growth (start over at protection of soil )
Used for grazing
Requires less water input than rainfed crops
In some areas people irrigate soils in arid regions which can increase the risk of soil salinization
Process of soils accumulating excess salt= soil salinization
Salt affected soils:
~7% of earths land area,
23% of cultivates area
50% of irrigated area
Can have an extremely bad effect on food
Alkaline soils: pH above 7
Saline soil: high concentration of soluble salts
in exchess of 4 deciSiements per meter
Salts commonly found in soils and natural water and their solubilty (mmolc L^-1)
Key point: carbonate and bicarbonate based salts are usually lower in solubilty than sulfate and chloride based salts
We can understand related process of saline lake formation ex: great salt lake
Due to inputs of water with dissolved salts
evaporation of water
absence of exit pathways for salts
repeat
Formation of saline soils through the addition of irrigation waer
Saline irrigated soils form from:
Inputs of water with dissolved salts
evapotranspiration of water
Absence of exit pathways for salts
repetition of this process
Even freshwater has small amounts of dissolved slats which are concentrated in the soil
Measuring salinity
Separately quantifying all the salts is too labor intensive and expensive
Therefore, we rely on bulk quantification of salts through
Total dissolved solids (TDS)
electrical conductivity (EC)
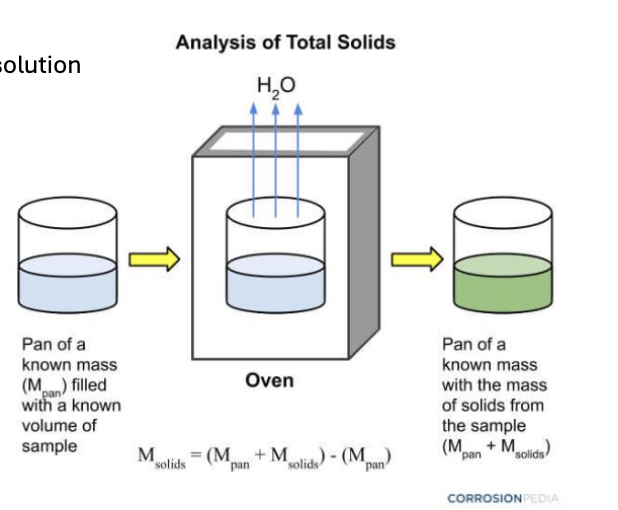
Total dissolved solids extraction process
Extraction of dissolved salts in aqueous solution
filtration to remove soil particles
Evaporation of water (shown in diagram)
Weighing of remaining soilds
Electrical conductivity, principle
More rapid than directly quantification of TDS
Based on principle of salt water a s a good conductor of electricity
More salts in solution —> greater electrical conductivity
Conductivity, practice
Mix distilled water with soil until it flows slightly
allow salts to dissolved overnight or half an hour
extract solution and measure ec with electrode
report ec reported in deciSiemens per meter
Describes abilty of soil to conduct electrical current
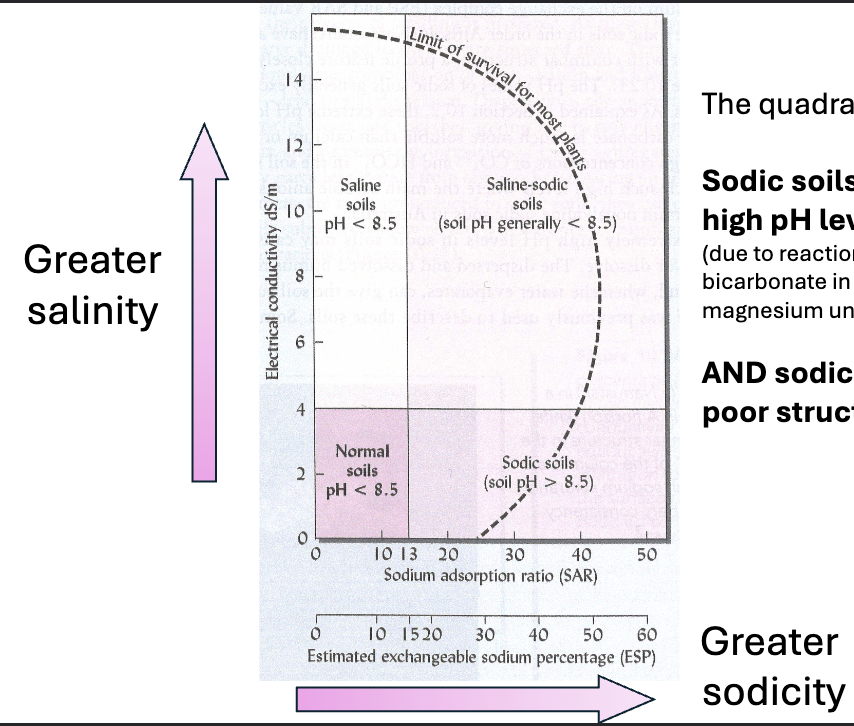
SODIC SOIL
The soluble salts are primarily sodium
Sodic soils are high in sodium as the dissolved salt
Higher in sodium because its lower in calcium
Soil sodicity can be quantified with the exchangeable sodium percent (ESP) shown here
OR with the sodium absorption ration (SAR)
SAR= {Na+}/(0.5{Ca2+}+0.5{Mg2+})
This quadrant of salinity and sodicity
Sodic soils can have particularly high pH levels
due to reactions of sodium with carbonate and bicarbonate in solution which calcium and magnesium undergo to much lesser extent )
AND sodic soils have particularly poor structure
The charge to hydrated radius of cations influences soil structure
Sodium has a slightly smaller hydrated radius than calcium or magnesium but only half of the charge
Lets imagine a couple of soil colloid particles van der waals forces can contribute to their aggregation
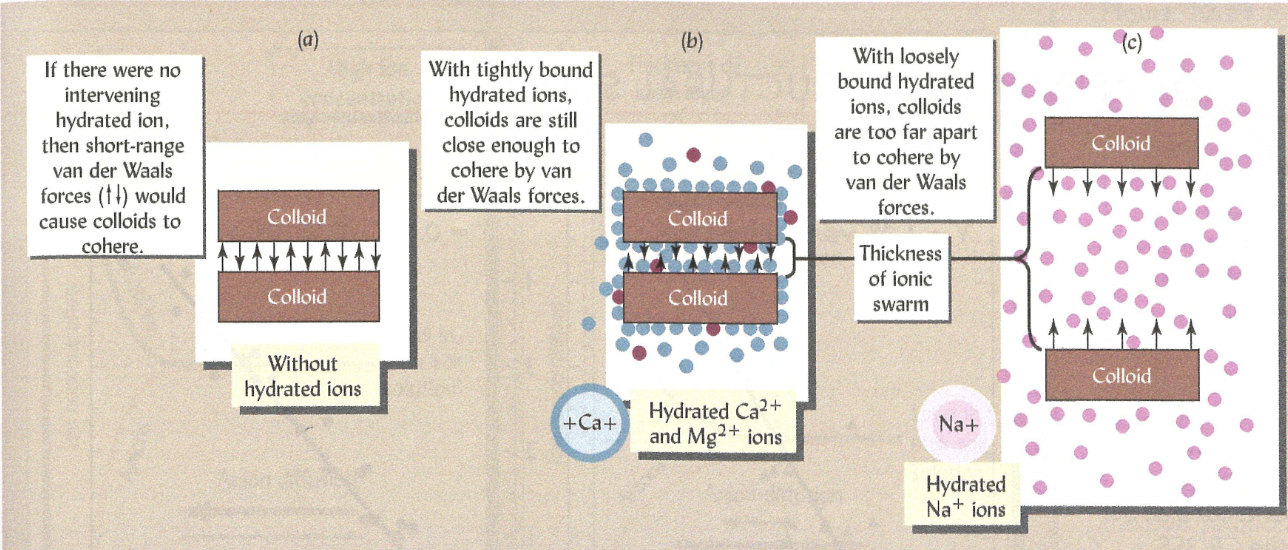
Sodic soils - consequences of poor structure
Forms a crust almost on top of soils
The charge of hydrated radius of cations influecnes soil structure
Sodium has slighly smaller hydrated radius than calcium but only half of the charge
will increase or decrease soil aggregation?
Sodic soils
COnsequences of poor structure
Flocculated (aggregated) vs dispersed strucure, flocculated can allow water to move, disperesed plugs soil pores and impede water movement
3 distinct causes of low permeability under sodic conditions
Dispersion
Clay particles seperate from one another rather than flocculating
Slaking
Aggregate disruption upon becoming wet —> clogging of soil pores
Swelling
Sodium enhances swelling expanding 2:1 clays
hich relationship would
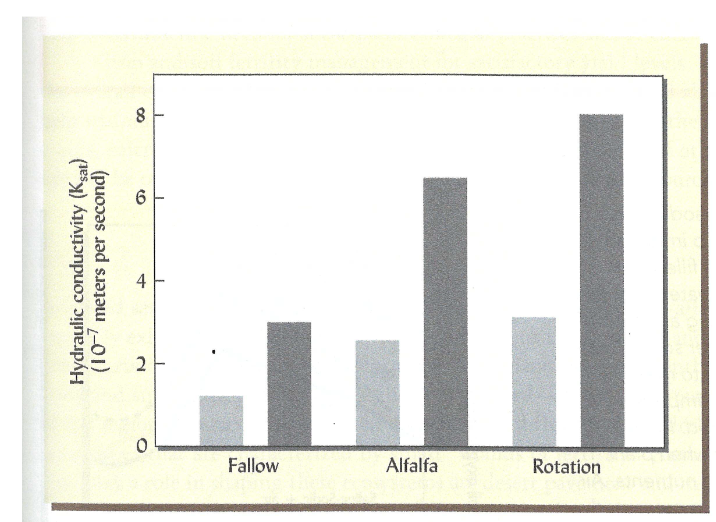
Which relationship would you expect between ESP and Ksat
ESP intereferes with Ksat
More ways salts can interfere with plant growth
Osmotic effects
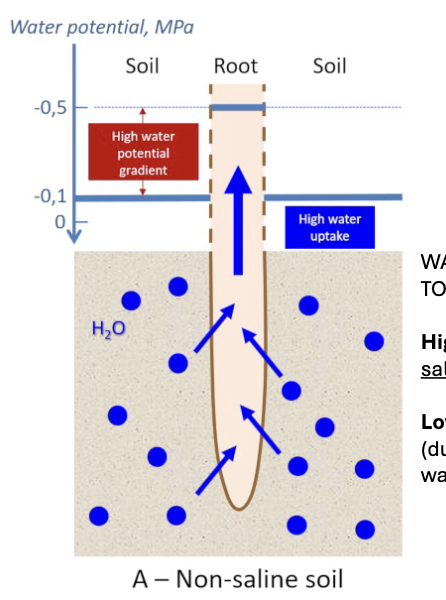
Water moves from high to lowe
Higehr potential in non saline soil solution
lower potential in plant root due to solites lowering water potential
Water in soil and plant converges in potential
making it more difficult for plant rooots to remove water from soil
Specific ion effects - what they are
Like mushroooms - some are harmless and some are deadly
Some ions are fine (CA2+ K+)
some ions cayse problems (Na+, CL-)
Specific ion effects:sodium
Sodium is a quasi essential element
Required for some but not all plants
neededby corn, sorghum, and oter tropical grasses
Excess sodium in soil can become toxic because Na competes for K+ which is an essential element
Are all saline soils also sodic soils?
False
Reclaimation strategies - saline soils
Cannot be reclaimed by chemcial amendments, conditioners, or fertilizers
Field can only be recliamed by removing salts from plant root zone
Opposing goals of irrigation
For refular irrigation: just apply enough water limitation on plant growth
for removing salts from root zone
Apply water in excess of what is needed for crop growth, so salts can move downward through soil profile and out of root zone
Efficacy of leeching
Reclaimation strategies - sodic soils
Application of gypsum - which contains calcium
calcium replaces sodium held in cation exchange on soil colloids
then soluble salt, NASO4 is formed, which can be easily leached away
Exam review
Aluminum toxicity
Aluminum is positively charged ion that can bind to the cation exchange capacity as soil becomes more acidic and the soil pH decreased
Aluminum displaces beneficial nutrients from the CEC
Cation exchange capacity
A soils ability to like exchange cations and how many positively charged ions a soil can hold
Expanding 2:1 clays have higher capacities
Protonation
The proces of adding protons (H+) to function groups on soil surfaces, which can change soil pH and charge, this occurs more often in acidic soils
Alfisols
Soil is rich in aluminum and iron
Argillic, kandic, or natric horizion
found in more wet soils
Ultisols
Strongly weathered acidic soils found in humic regions
HIgh in pH and Al3+
Found in more temperate areas
You uncover archives of ancient civilization
Instead of 12 soil orders, they group soils into 3 categories based on base saturation
Low base saturation
Medium base saturaton
HIgh base saturation
Describe extent of soil weathering for each of these three soil orders
As soils beccome more weathered, base saturation goes down
so a more sautrated soil will be less weathered.
Mollisols