What To Memorize: Exam 2
Chapter 4
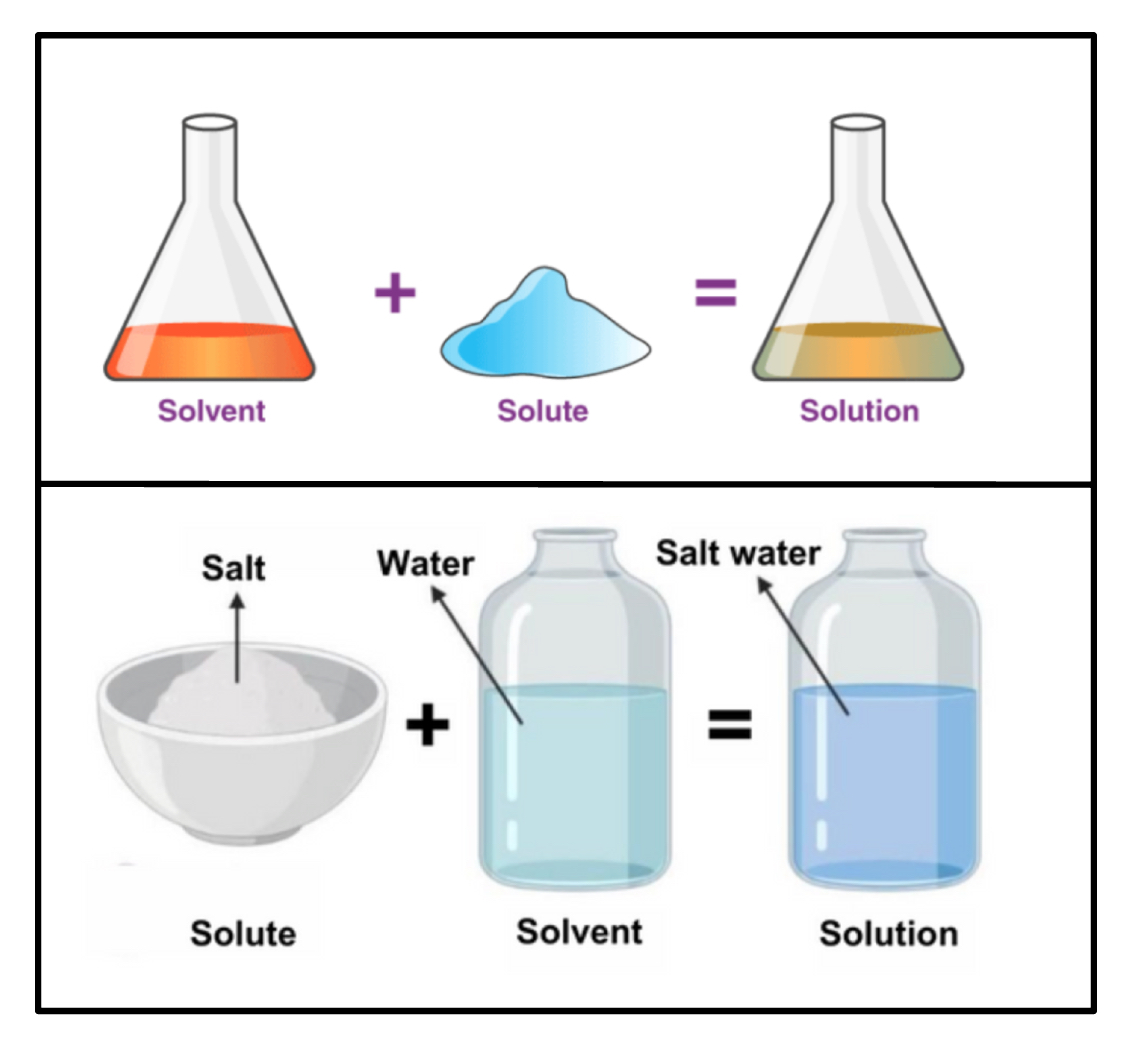
Solute vs. Solvent vs. Solution
Solute: The substance (solid) that is dissolved in the solvent.
Solvent: The liquid that dissolves the solute, present in greatest abundance.
Solution: A homogeneous mixture of two ore more pure substances.
When water is the solvent, the solution is called an aqueous solution.
Molarity Solution (M) = (moles solute)/(L solution)
M=\dfrac{mol}{V}
Volume is in Liters
Dilution Equation: McVc=MdVd
Mc and Md = molarity of the concentrated solution
Vc and Vd = the volumes of the two solutions
c = concentrated
d = dilute
Strong Acids
Hydrochloric Acid (HCl)
Hydrobromic Acid (HBr)
Hydroiodic Acid (HI)
Chloric Acid (HClO3)
Perchloric Acid (HClO4)
Nitric Acid (HNO3)
Sulfuric Acid (H2SO4)
Weak Acids
Hydrofluoric Acid (HF)
Acetic Acid (CH₃COOH)
Strong Bases
Group 1A metal hydroxides
Heavy group 2A metal hydroxides (Ca(OH)2) and down
Assigning Oxidation States
The oxidation state of an atom in a free, uncombined element = 0
The sum of oxidation numbers of all atoms must equal the total charge (neutral = 0, ion = charge)
In compounds,
Group 1A = +1 (not including H)
Group 2A = +2
B and Al = +3
F = -1
In compounds, H = +1
In compounds, O = -2
In their binary compounds with metals
Group 5A = -3
Group 6A = -2
Group 7A = -1
Higher order rule takes precedence
Balancing Redox Reactions
Divide the reaction into half reactions
Balance all atoms in half reactions except “H” and “O”
Balance “O” by adding H2O
Balance “H” by adding H
Balance each half reaction electronically by adding e-
Multiply each half reaction by an integer to get electrons in each half reaction to be the same
Next step depends on the type of solution:
Acidic Solutions: Combine the two half reactions, canceling anything that is the same on both sides of the arrow
Basic Solutions: Add OH- to both sides of the reaction to balance the H+. H+ + OH- will yield H2O, then add the two half reactions
Chapter 5
Formulas
Change in internal enthalpy: \Delta E=E_{f}-E_{0}
Exchange of energy between system and surroundings: \Delta E=q+\omega
q = heat
w = work
If \Delta E >0 = Endothermic (the system absorbs energy from its surrounding in the form of heat)
If \Delta E <0 = Exothermic (the system releases energy in the form of heat)
Work: PV or -PV
P = pressure
V = volume
Enthalpy: H=E+PV
Change in Enthalpy: \Delta H=\Delta E+P\Delta V
Enthalpy of Reaction: \Delta H_{rxn}=H_{products}+H_{reactants}
Chapter 6
Formulas
Specific of light: c=\lambda v
\lambda = wavelength
v = frequency
Constant for speed of light: c=3.00\times 10^{8}m/s
Energy is proportional to frequency: E=hv
h = plank’s constant
v = frequency
Plank’s constant: h: 6.626\times 10^{-34}J\cdot s
Reminders
Fahrenheit: F = \dfrac{9}{5}\left( °C+32\right)
Celsius: C = \dfrac{5}{9}\left( °F-32\right)
Kelvin: K = °C+273
 Knowt
Knowt
