2.4.0 - Polarity
Introduction
- ^^Polarity^^ - “The distribution of electrical charge over the atoms connected by the bond” (x)
- The difference in electronegativity between elements in a molecule determines whether the overall molecule is polar or nonpolar.'
- Difference in electronegativity causes partial positive (𝛿+) and negative charges (𝛿-).
- A molecule with two of the same element will always result in a nonpolar bond (i.e, they are not polar opposites in terms of their ability to attract electrons)
- Polarity of molecule affected by structure - existence of lone pairs will affect shape and polarity.
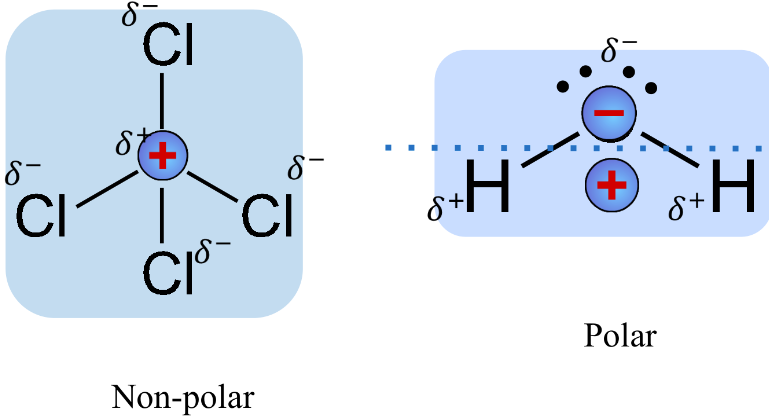
Shape & Polarity
- Shape determines the distribution of the partial charges within the molecules.
- ^^Dipole^^ - molecule where the ends have opposing charges.
- Polar if:
- Distinctive regions of charges
- Non-symmetrical shapes (frequently)
- Non-polar if:
- No distinctive regions of charges
- Symmetrical shapes (frequently)
Determining Polarity of a Molecule
Assign partial charges (electronegativity) to all atoms within the molecule, based on the “flow of electrons'“
- e.g in barium fluoride (BF3), fluorine, the most electronegative element, will have a partial charge of (𝛿-) as the negatively charged electrons from barium will ‘drift’ towards it. Barium will have a partial charge of 𝛿+.
Determine whether each bond is polar or non polar. If the molecule has no polar bonds or it is symmetrical, it is nonpolar.
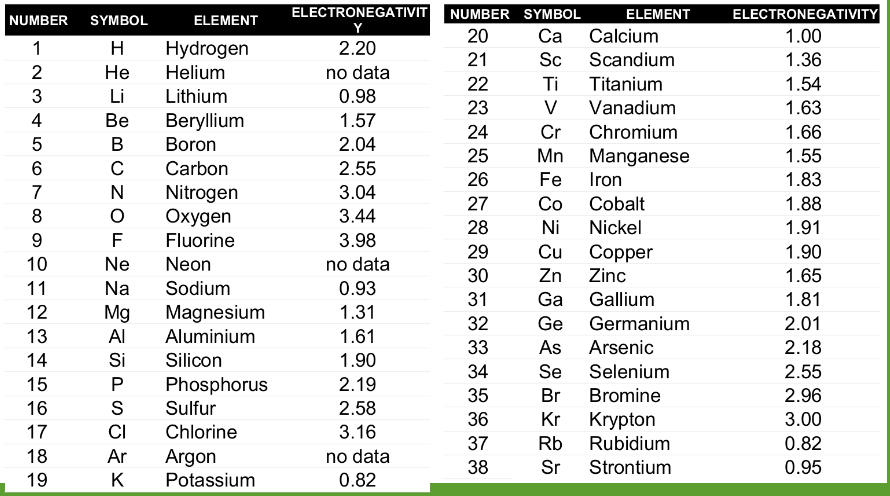
Electronegativity to determine Polarity
- Electronegativity used to determine polarity of a molecule.
- The greater the difference between electronegativity values, the more polar it is.
| Approx. Electronegativity Difference | Type of Bond | Example |
|---|---|---|
| 0.0 - 0.4 | Covalent (non polar) | H-H |
| 0.4 - 1.0 | Covalent (moderately polar) | H-Cl |
| 1.0 - 2.0 | Covalent (highly polar) | H-F |
| >2.0 | ionic |