Titrations
What are titrations?
Titration is a laboratory method for determining the concentration of a solution by reacting it with a solution of known concentration, known as the titrant.
The process involves adding the titrant to the solution being analysed until the reaction reaches its endpoint, often indicated by a colour change or a measurable physical property.
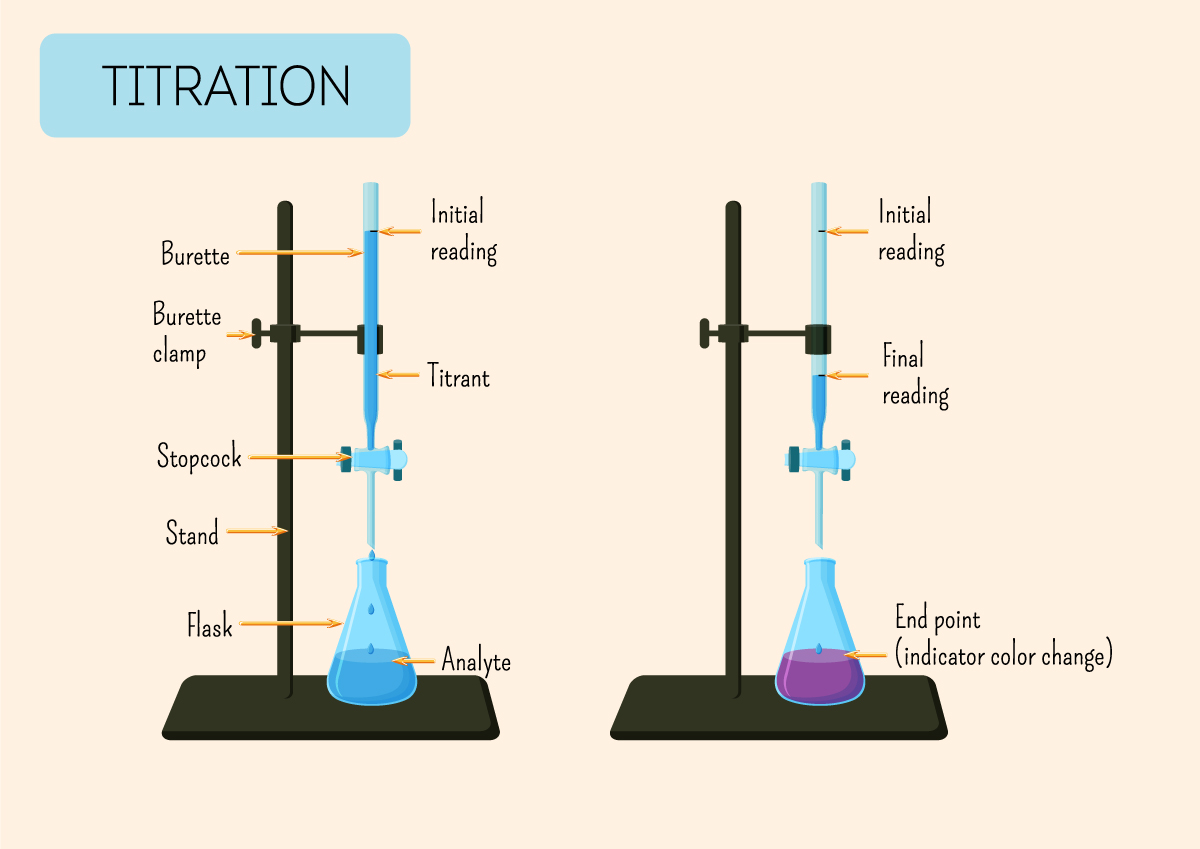
First titration is an estimate
Concordant titres are results that are within 0.10 cm³ of each other
Common Questions to note
What is a meniscus within a burette?
The meniscus is the curve seen at the surface of a liquid in a burette, which is caused by surface tension.
It is crucial to read the volume of the liquid at the bottom of the meniscus to ensure accurate measurements during titrations.
Why is the burette rinsed with deionised water before use?
Rinsing the burette with deionised water ensures that any contaminants or residues from previous experiments are removed, preventing interference with the accuracy of the titration results.