Biology 1 - Mod 2 pH Scale, Chemical Reactions, and Macromolecules
Water molecules can react to form ions.
H2O <> H+ and O+ = Water <> hydrogen and hydroxide ion
The number of negative and positive ions produced is equal, so the water remains neutral. But, the charge of water enables it to act as a universal solvent.
pH Scale
Indicates the concentration of H+ ions in solutions. The scale ranges from 0-14. Increases by values of 10 (3 is 10x more acidic than 4). At 7, the solution is neutral. Below 7, it is acidic. Above 7, it is basic.
Acids
A compound that forms H+ ions in solution.
pH value below 7
Strong acids pH 1-3
Examples: Hydrochloric acid in stomach
Bases
A compound that produces hydroxide atoms in solution.
pH value above 7
Strong bases 11-14
Examples: Soap/lye, cleaning products
Buffers
Weak acids or bases that can react with strong acids or bases to prevent changes in pH.
Many buffers are in the human body to help maintain homeostasis.
Macromolecules
Carbohydrates
Function: energy storage, forms cell membranes, chemical messengers, protection/insulation
Monomer (subunit): Monosaccharides (glucose + fructose)
Examples: glucose (sugar), starch, glycogen, cellulose
Lipids
Function: energy storage
Monomer (subunit): Glycerol + fatty acids
Examples: Fats, Oils, Waxes, Cholesterol, Vitamins, Phospholipids (cell membrane)
Proteins
Function: storage, transport, regulatory, movement, structural, enzymes
Monomer (subunit): Amino Acids
Examples: Keratin (hair, nails), muscles, silk, nuts, beans, albumin, hemoglobin, insulin
Nucleic Acids
Function: carries genetic information to make proteins
Monomer (subunit): Nucleotides
Examples: DNA, RNA
Chemical Reactions
| reactants | products | name |
|---|---|---|
| C6H206 | 6 H2O + 6 CO2 | cell respiration |
| 6 H20 + 6 CO2 | C6H2O6 + 6 O2 | photosynthesis |
Enzyme: a protein catalyst that can decrease the energy border (speeds up a reaction).
Activation energy is lower with an enzyme activation.
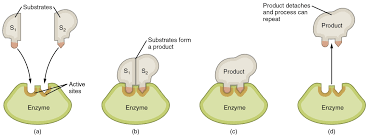
The Lock and Key Model
The enzyme is unchanged and can repeat the process.
Enzyme activity is influenced by
- temperature
- salt concentration
- pH
- denaturing
Denaturing is the process in which an enzyme changes shape, leading it to no longer work.