Chapter 13: Acids and Titrations
13.1-Acids and Bases
The pH scale goes from 0 to 14
- The pH scale is a measure of how acidic or alkaline a solution is
- The lower the pH of a solution, the more acidic it is
- The high the pH of a solution, the more alkaline it is
- A neutral substance has pH 7
You can measure the pH of a solution
- An indicator is a dye that changed colour depending on whether it’s above or below a certain pH
- Some indicators contain a mixture of dyes meaning they gradually change colour
- Called wide range indicators for example universal indicator
- A pH probe attached to a pH meter can also be used to measure pH electronically
- The probe is placed in the solution you are measuring and the pH is given on a digital display as a numerical value meaning its more accurate
Acids and bases neutralise each other
- An acid is a substance that forms aqueous solution with a pH of less than 7
- Acids form H+ions in water
- A base is a substance with a pH greater than 7
- An alkali is a base that dissolves in water to form a solution with a pH greater than 7
- Alkalis form OH-ions in water
- The reaction between acids and bases is called neutralisation:
- Acids + base - salt + water
- Neutralisation between acids and alkalis can be seen in terms on H+ and OH-ions:
- H+ + OH- - H20
- When an acid neutralises a base the products are neutral
- An indicator can be used too show that a neutralisation reaction is over
- Neutralisation reactions of strong acids and alkalis can be used to calculate the concentration of an acid or alkali by titration, there is more about this technique
13.2-Titrations
Acid-alkali titrations
- The concentration of an acid or alkali can be calculated by carrying out an experiment called a titration.
- Materials
- The apparatus needed includes:
- a pipette to accurately measure a certain volume of acid or alkali
- a pipette filler to use the pipette safely
- a conical flask to contain the liquid from the pipette
- A burette
Apparatus needed to carry out a titration
Method
- This is an outline method for carrying out a titration in which an acid is added to alkali.
- Use the pipette and pipette filler to add 25 cm3 of alkali to a clean conical flask.
- Add a few drops of indicator and put the conical flask on a white tile.
- Fill the burette with acid and note the starting volume.
- Slowly add the acid from the burette to the alkali in the conical flask, swirling to mix.
- Stop adding the acid when the end-point is reached (the appropriate colour change in the indicator happens). Note the final volume reading.
- The same method works for adding an alkali to an acid – just swap around the liquids that go into the conical flask and burette.
13.3-Strong Acids and Weak Acids
Strong and weak acids
- Strong acids dissociate fully in water to produce the maximum number of H+ ions.
- This means if you had one mole of hydrochloric acid (HCl) molecules, they would all ‘split’ to form one mole of H+ ions and one mole of Cl– ions.
- Weak acids, such as ethanoic acid (CH3COOH), do not fully dissociate.
- In fact, about only one per cent of ethanoic acid molecules split up to form H+ ions and CH3COO– ions at any one time.
- This means that the pH values of strong acids are lower than that of weak acids, which explains why the rate of reaction of strong acids with substances (such as metals, metal carbonates etc) is higher than that of weak acids.
- This also explains why the temperature rise during a reaction with strong acids is higher than that of weak acids.
Concentrated and dilute acids
- Weak and strong should not be mistaken for dilute and concentrated.
- A dilute acid has the acid molecules mixed with a large amount of water, so that there is only a low concentration of H+ ions.
- Concentrated acids have little to no water molecules mixed with the acid molecules, meaning the concentration of H+ ions is high.
13.4-Reactions of Acids
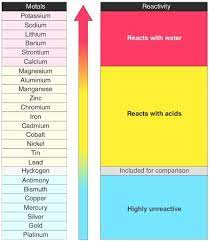
Reactions of acids with metals
- Only metals above hydrogen in the reactivity series will react with dilute acids
- The more reactive the metal then the more vigorous the reaction will be
- Metals that are placed high on the reactivity series such as potassium and sodium are very dangerous and react explosively with acids
- When acids react with metals they form a salt and hydrogen gas:
- The general equation is:
- metal + acid ⟶ salt + hydrogen
Acid-Metals Reactions Table
- In general, we can summarise the reaction of a metal that forms a +2 ion as follows:
Reaction of acids with oxides & hydroxides
When an acid reacts with an oxide or hydroxide, a neutralisation reaction occurs
Metal oxides and metal hydroxides act as bases
In all acid-base neutralisation reactions, a salt and water are produced:
- acid + base ⟶ salt + water
The identity of the salt produced depends on the acid used and the positive ions in the base
Hydrochloric acid produces chlorides, sulfuric acid produces sulfate salts and nitric acid produces nitrates
The following are some specific examples of reactions between acids and metal oxides / hydroxides:
- 2HCl + CuO ⟶ CuCl2 + H2O
- H2SO4 + 2NaOH ⟶ Na2SO4 + 2H2O
- HNO3 + KOH ⟶ KNO3 + H2O
In general, we can summarise the reaction of metals and bases as follows:

Reactions of Acids with Metal Carbonates
- Acids will react with metal carbonates to form the corresponding metal salt, carbon dioxide and water
- These reactions are easily distinguishable from acid – metal oxide/hydroxide reactions due to the presence of effervescence(the bubbles in a fizz) caused by the carbon dioxide gas
Practice Questions
- In a titration of sulfuric acid against sodium hydroxide, 32.20mL 32.20mL of 0.250MNaOH0.250MNaOH is required to neutralize 26.60mL26.60mL of H2SO4H2SO4. Calculate the molarity of the sulfuric acid.
H2SO4(aq)+2NaOH(aq)→Na2SO4(aq)+2H2O(l)H2SO4(aq)+2NaOH(aq)→Na2SO4(aq)+2H2O(l)
- Solution
- Step 1: List the known values and plan the problem.
- Molarity NaOH=0.250MNaOH=0.250M
- Volume NaOH=32.20mL NaOH=32.20mL
- Volume H2SO4=26.60mLH2SO4=26.60mL
- Unknown
- First determine the moles of NaOH NaOH in the reaction. From the mole ratio, calculate the moles of H2SO4H2SO4 that reacted. Finally, divide the moles of H2SO4H2SO4 by its volume to get the molarity.
- Step 2: Solve.
- mol NaOH=M×L=0.250ml