Detailed Overview of Bacterial Respiration and Pathogenicity in E. coli
Introduction to Bacterial Respiration
Bacterial respiration refers to the process through which bacteria convert nutrients into energy, primarily using aerobic and anaerobic pathways. Central to this process is the electron transport chain (ETC), which plays a crucial role in ATP production.
Energy Metabolism
Within bacteria, reduced electron carriers like NADH and FADH2 are vital for energy metabolism. These carriers feed electrons into the respiratory chain, facilitating the movement of electrons along various respiratory complexes. This movement is essential, as it allows for the production of significant amounts of ATP. Bacteria obtain ATP and additional reduced electron carriers through pathways such as glycolysis and the Krebs Cycle, both of which contribute electrons to the respiratory chain.
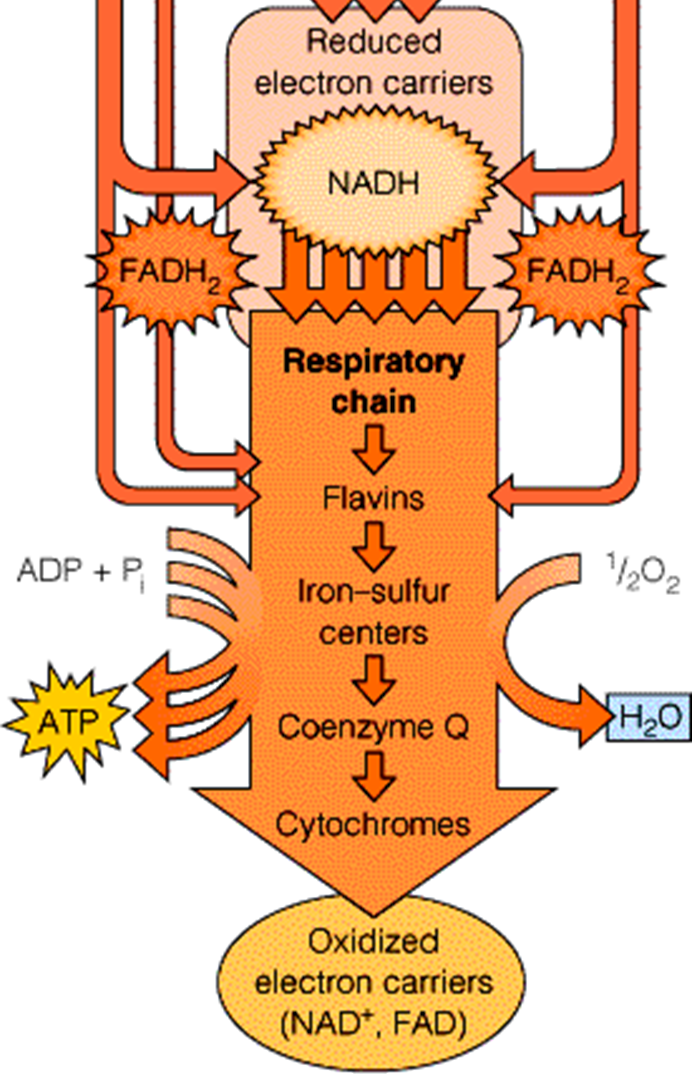
Types of Respiration
Aerobic Respiration
Aerobic respiration is characterized by the transfer of electrons from donor molecules (for example, NADH) to oxygen, which is subsequently reduced to water. This process is energetically downhill and is coupled with the translocation of ions, generating an electrochemical gradient critical for ATP production.
Anaerobic Respiration
Anaerobic respiration involves the transfer of electrons to molecules other than oxygen or to ionic species. The mechanisms include several types of oxidoreductases that facilitate this transfer in environments devoid of oxygen.
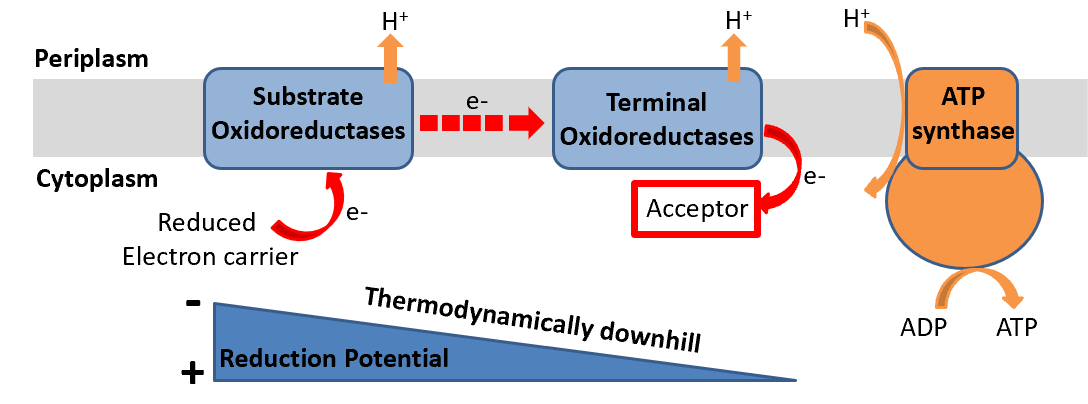
Fermentation
Fermentation is a metabolic process that reduces endogenous organic compounds to produce various products such as lactate or acetate. It serves as a way to maintain redox balance in the absence of respiration.
Electron Donors and Acceptors
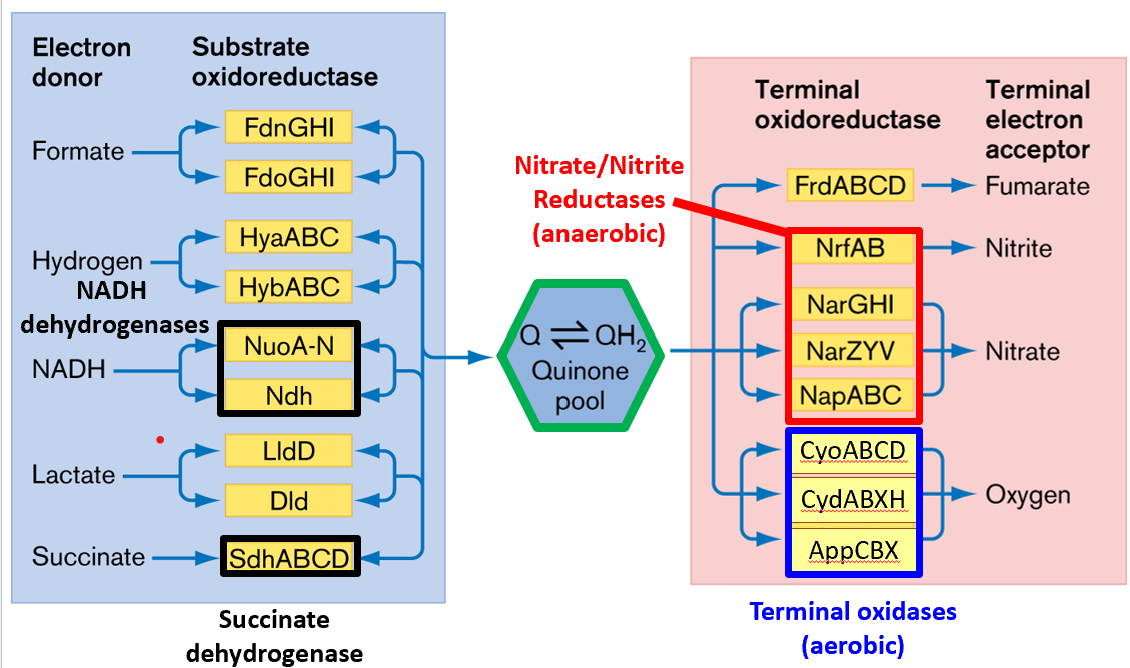
Bacteria possess numeroua alternative electron donors (blue on left) and acceptors (red on right). Bacteria, particularly E. coli in this example, utilise an array of alternative electron donors and acceptors. Common examples include:
NADH Dehydrogenases (aerobic and anaerobic forms)
Terminal Oxidases like CyoABCD (for aerobic conditions) and NarGHI (for nitrate reduction)
Nitrate/Nitrite Reductases that function under anaerobic conditions.
Electron Transfer Routes
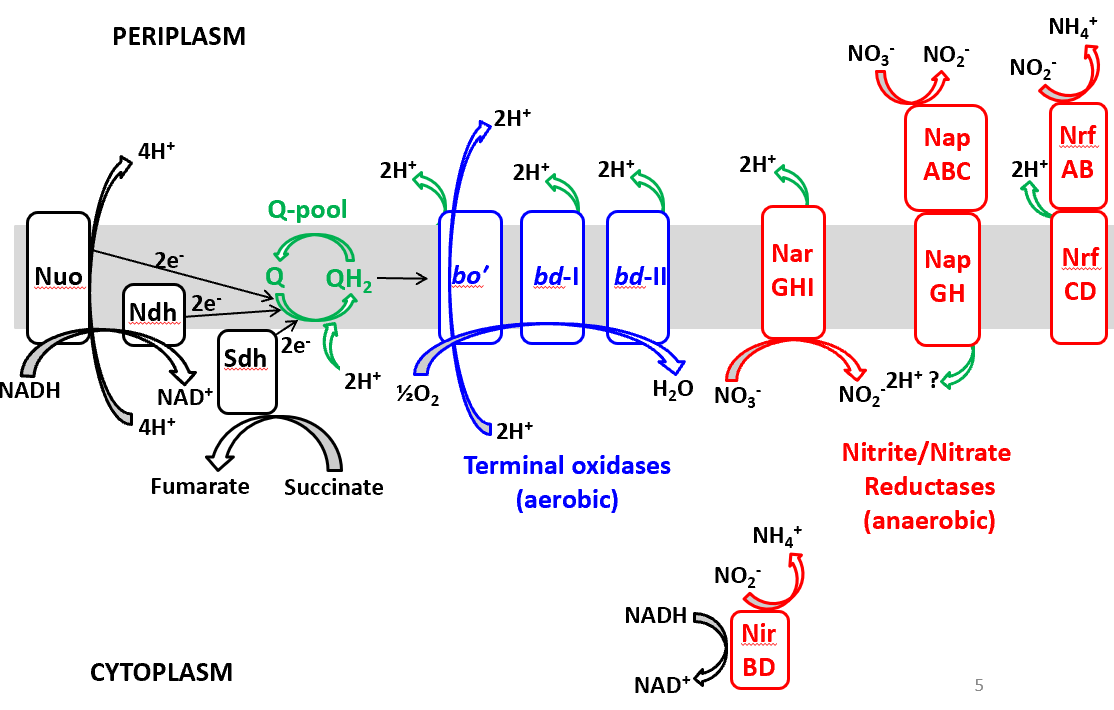
The electron transfer system in E. coli comprises various complexes that interact with reduced carriers like NADH, contributing to electron pools such as UQ (ubiquinone) and MQ (menaquinone). Nuo are is the entry part. Nuo and Ndh are both NADH hydroylases.
Key components highlighted in the image may include:
Nuo: This complex is primarily involved as the entry point for NADH in the electron transfer process. It functions as an NADH dehydrogenase.
Ndh: Similar to Nuo, Ndh also acts as an NADH hydrolyase that contributes to the pool of electrons in the respiratory chain.
Sdh: This complex further integrates within the ETC, carrying out important roles for electron handling and energy conservation.
The terminal oxidase section is the point where the electrons are finally transferred to oxygen or other terminal electron acceptors in the respiratory chain. Terminal oxidases are crucial for aerobic respiration as they catalyze the reduction of oxygen to water, which is the endpoint for electron flow through the chain.
The nitrite/nitrate reductase section of the image highlights the role of specific complexes, namely NrfABCD and NarGHI, that facilitate anaerobic respiration in E. coli. These reductases are crucial in environments where oxygen is absent, allowing the bacteria to utilize alternative electron acceptors for energy production.
Nitrate Reductase (NarGHI): This complex is responsible for the reduction of nitrate (NO₃⁻) to nitrite (NO₂⁻). It plays a key role in anaerobic respiration by providing an alternative pathway for electron transfer that supports ATP synthesis when oxygen is unavailable. NarGHI operates efficiently in low-nutrient conditions and contributes to the nitrogen cycle in the environment.
Nitrite Reductase (NrfABCD): This complex further reduces nitrite to ammonia (NH₃) or other nitrogen compounds. Its action is essential in maintaining redox balance in the microbial community and allows E. coli to adapt to fluctuating oxygen levels. NrfABCD is particularly important in conditions rich in nitrite, enhancing bacterial survival and adaptability.
Electron shutting in the respiratory chain of E. coli
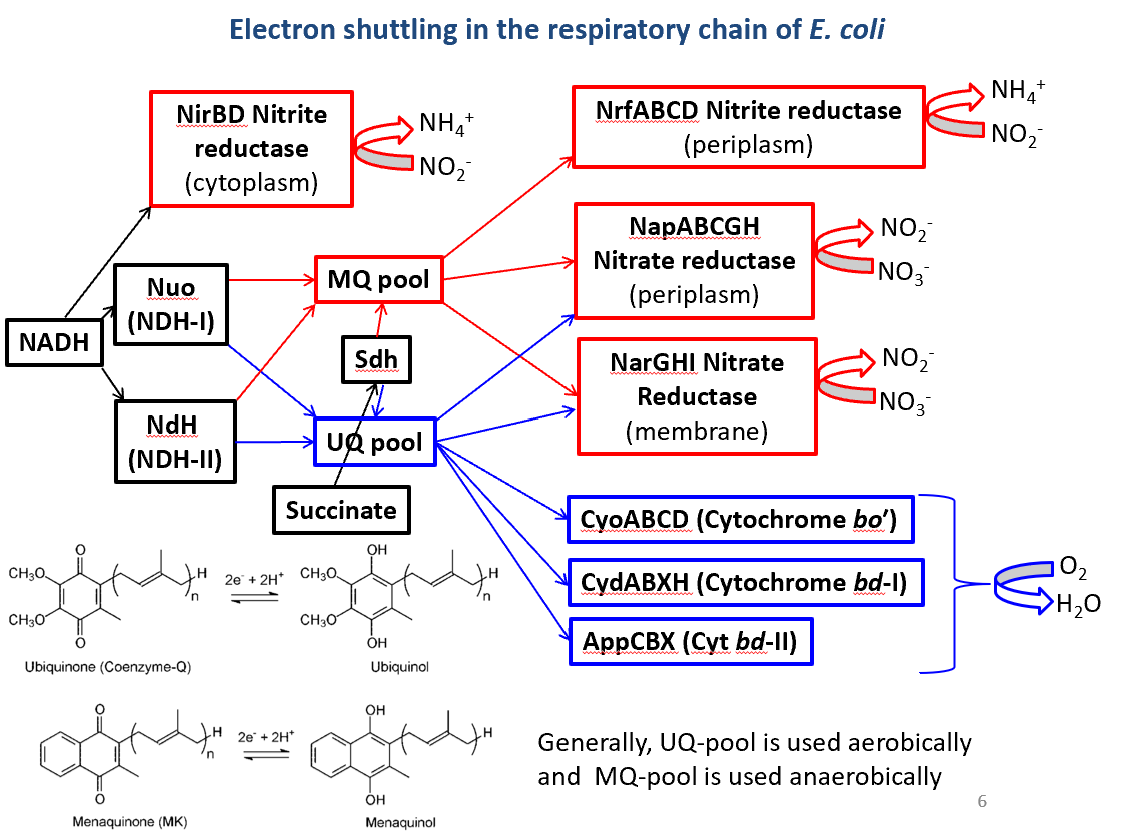
Here are the key components and their roles:
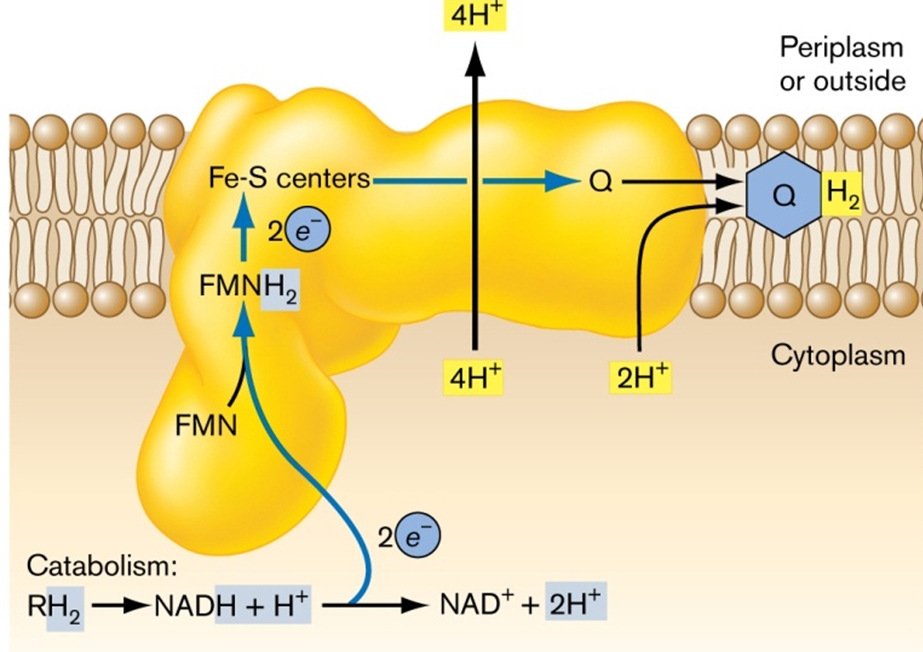
NADH Dehydrogenase (NDH-I): This complex acts as a proton pump. It transfers electrons from NADH into the electron transport chain (ETC) while also translocating protons across the membrane, contributing to the establishment of a proton gradient essential for ATP synthesis.
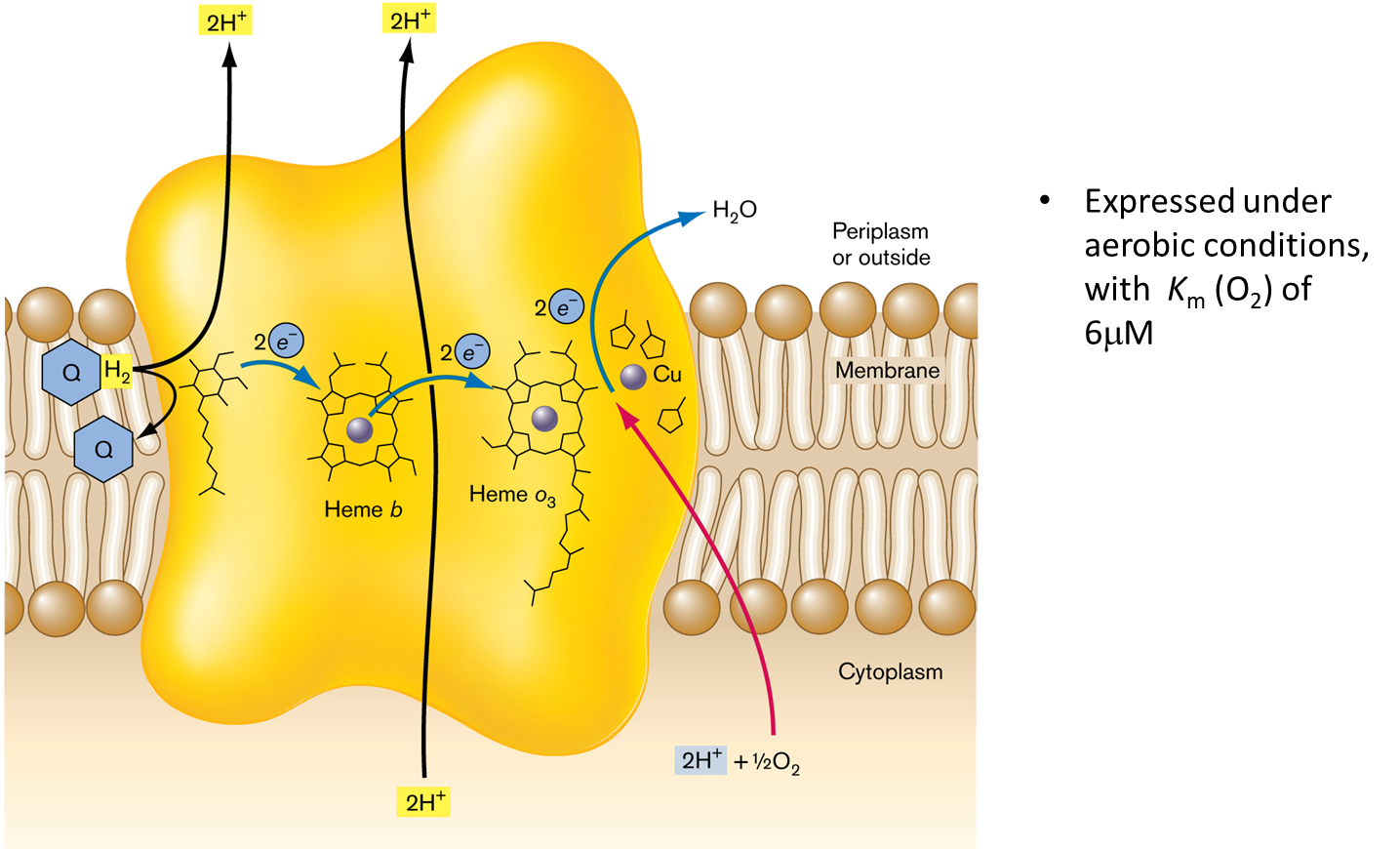
Cytochrome bo′: Similar to NDH-I, this complex functions as a proton pump with specific affinities for different oxygen levels, facilitating efficient proton translocation.
Nitrate Reductase (NarGHI) and Nitrite Reductase (NrfABCD): These complexes are involved in the reduction of nitrate and nitrite under anaerobic conditions, respectively. They play a significant role in energy production in the absence of oxygen, allowing E. coli to thrive in low-oxygen environments.
During the process of electron transfer, the conservation of energy occurs through the formation of a proton gradient, which is ultimately harnessed for ATP synthesis and is critical for the bacterium's energy metabolism. Understanding these electron transfer systems is essential for comprehending how E. coli adapts to varying environmental conditions and efficiently generates energy.
Energy Conservation
During the transportation of electrons, some complexes conserve energy by forming a proton gradient, which is employed for ATP synthesis. Notable electrogenic respiratory complexes include:
NADH Dehydrogenase (NDH-I)
Cytochrome bo′
Nitrate Reductase NarGHI
Nitrite Reductase NrfABCD
Specific Complexes
NADH Dehydrogenase (NDH-1)
NDH-I operates as a proton pump, transferring electrons from NADH and contributing to a higher number of H+ translocated across the membrane per electron pair. four channels transfer protons across the membrane: scalar translocation. NDH-1 is expressed maximally in areobic conditions but, can reduce both UQ or MQ. as seen in the image below, two electrons are transferred from NADH to FMN, a very efficent way of making the proton gradient
Cytochrome bo′
This complex also functions as a proton pump but has specific affinities for oxygen levels. It shows distinct behaviors in scalar and vectorial proton translocation. It has 4 subunits (CyoABCD). This pump is expressed under areobic conditions with Km (O2) of 6mM.
Cytochrome bd-I
has 4 subunits. Expressed under low oxygen conditions (Km (Os) = 270nm). This cytochrome is vital for maintaining functions in environments that are low in oxygen but rich in nitric oxide (NO). Cytochrome oxidises ubiquinol. Only vectorial proton translocation. IT IS NOT A PUMP.
Nitrite and Nitrate Reductases (NrfABCD and NarGHI)
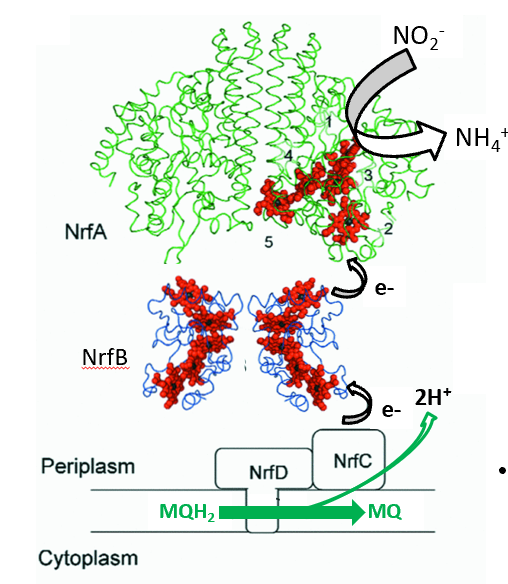
MarGHI is express maximally under anaerobic conditions in the presence of nitrate. These complexes play a crucial role in the reduction of nitrite and nitrate-laden environments, contributing to the regulation of ATP synthesis under anaerobic conditions. They operate under low nutrient conditions, emphasizing the adaptability of bacterial respiratory processes. NOT A PUMP, only contirbutes to PMF via vectorial translocation.
Nitrite reductase catalyses the six electron reduction of nitrite to ammonia. it is expressed under anaerobic/nicroaerobic conditions in the presence of nitrate and nitrite.
NrfA and NrfB together make up a 20 haem complex (site of NO2 reduction). the mediator between the NrfCD complex and NrfA is the penthaem NrfB protein. they form a reversible complex and contribute to PMF via vectorial translocation.
Pathogenicity of E. coli
Pathogenic E. coli strains significantly impact human health, with rising cases of bacteremia and antibiotic resistance. Notably, the globally spread O25:H4-ST131 strain is linked to various infections, raising concerns about treatment strategies.
Role of Nitric Oxide (NO)
NO functions as a respiratory inhibitor produced by host immune responses, presenting challenges to bacterial survival. Pathogens have developed mechanisms, notably through the NrfABCD complex, to detoxify this radical, allowing them to withstand elevated NO levels during infection.
Conclusion
These notes provide an overview of the fundamental aspects of bacterial respiration, focusing on the diverse electron carriers and pathways that allow bacteria to effectively generate energy and adapt to different environments. Additionally, they underline the clinical significance of respiratory mechanisms in pathogenic E. coli, highlighting potential strategies for targeting these bacteria in therapeutic contexts. Understanding the interplay between respiration and antibiotic resistance will be key as we develop methods to combat multidrug-resistant infections.