Chemistry HL 3.2 Periodic Trends
Periodic Trends
things that repeat in the periodic table
atomic/ionic radii, electonegativity, ionization energy → physical properties that explain the chemical properties
Effective Nuclear Charge
nuclear charge = number of protons = atomic number
inner electrons “shield” the nuclear charge that comes from the nucleus from the outer electrons
the outer electrons do not feel the full attracton of the nuclear charge as they are shielded and repelled by the inner electrons
the charge they do experience is the “effective charge” which is less than the full nuclear charge
outer electrons = effective nuclear charge
example: 11 protons 10 inner electrons (Na) → 11-10 = +1 effective nuclear charge
outermost shell has only 1 electron so 11 total electrons but 10 inner electrons
effective nuclear charge increases with atomic number as you go across a period (left to right) as there is no change in the number of inner electrons, as all atoms have a noble gas structure
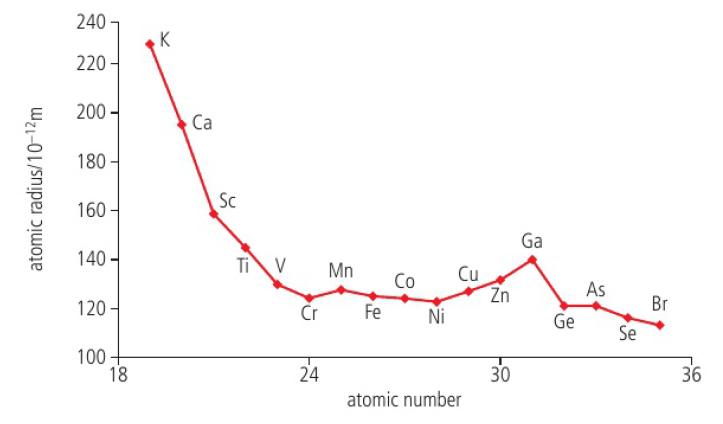
Atomic radius
to find size of an atom, measure distance between two nuclei in a bonded form
used as a diameter, so cut that measurement in half to find the atomic radius
increasing nuclear charge causes the atom to decrease in size
if you go down a group the size increases because the number of occupied electron shells (given by the periodic number) increases
if you go across a period the size decreases because they go in the same energy level when you go across and the electrostatic forces are stronger
higher electron energy level = bigger
distribution of the charge / electrostatic forces affects the atomic radius
decreased pull = bigger atom, outer electrons go further away from the nucleus
Ionic radius
positive ions are smaller, negative ions are bigger
positive → more pull distributed among each electron as one or more electron leaves
negative → increased electron repulsion between electrons in the outer principal energy level causes the electrons to move further apart and so increases the radius of the outer shell
ionic radii decreases from Groups 1-14 for positive ions even though they have the same electron configuration
increase in nuclear charge with atomic number across the period causes increased attraction between the nucleus and the electrons (pulls the outer shell closer to the nucleus)
ionic radii decreases from Groups 14-17 for negative ions even though they have the same electron configuration
increase in nuclear charge across a period
positive ions are smaller than the negative ions, as the former have only two occupied electron principal energy levels and the latter have three
discontinuity in the table (1-14, 14-17)
ionic radii increases down a group as the number of electron energy levels increase
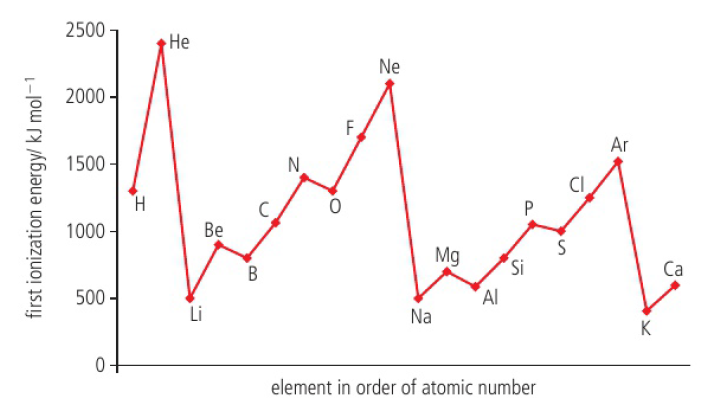
Ionization energy
measure of attraction between nucleus and outer electrons
increases across a period
increase in effective nuclear charge causes an increase in the attraction which makes the electrons more difficult to remove
decreases down a group
electron removed is from the energy level furthest from the nucleus, and although nuclear charges increases, the effective nuclear charge is about the same because of the shielding effect, but the distance between the outer electrons and the nucleus is increased so the attraction is weaker
higher energy level / higher energy (p>s) means ionization energy is smaller as the increased energy makes the orbital more unstable and more susceptible to “pulling” an electron out
gaseous atoms
removing one electron
energy input (all positive values)
endothermic
A (g) → A+ + e-
Electron affinity
First electron affinity of an element
energy change when one mole of electrons is added to one mole of gaseous atoms to form one mole of gaseous ions
A (g) + e- → A-
exothermic
added electron is attracted to the positvely charged nucleus
Second electron affinity of an element
defined similarly to first electron affinity
example: O-(g) + e- → O2-(g)
process is endothermic as the added electron is repelled by the negatively charged oxide ion and energy needs to be available for this to occur
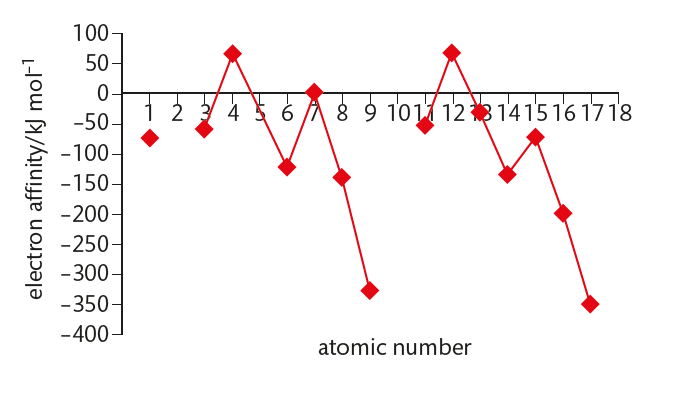
negative values = exothermic, positive = endothermic
similar to ionization energy graph, but displaced to the right by one and inverted
electron affinity minimum values = Group 17, ionization energy maximum values = Group 18
Group 17 elements have incomplete outer energy levels and a high effective nuclear charge of approximately +7 so attraction is strongest
Group 1 metals have lowest effective nuclear charge of approximately +1 and so attraction is weakest
Group 2 and 5 elements are the maximum because they have ns² electron configurations, so the added electron must be put into a 2p orbital which is further from the nucleus and so experiences reduced electrostatic attraction due to shielding from electrons in the ns orbital
Group 15 elements have the configuration ns2np3 so the added electron must occupy a p orbital that is already singly occupied (arrow diagram)
the attraction between the electron and atom is less than expected as there is increased inner-electron repulsion (exothermic only for nitrogen)
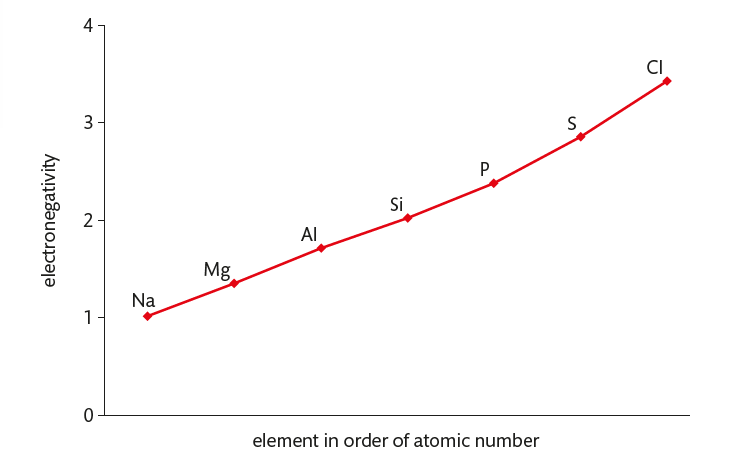
Electronegativity
attraction/pull an atom has on the electron pairs it shares with another atom in a covalent bond (attraction between nucleus and electrons)
covalent bond (shared electron pairs) → not equally shared
similar to ionization energy
both measure attraction between the nucleus and its outer electrons - in this case bonding electrons
same trends (period and group)
differences
ionization energy can be measured directly and are a property of gaseous atoms
elements with high electronegativities have the most exothermic electron affinities
electron affinity is a property of isolated gaseous atoms
electronegativity is a property of an atom in a molecule
derived indirectly from experimental bond energy data
increases across a period
increase in nuclear charge resulting in an increased attraction between nucleus and bond electrons
decreases down a group
electrons furthest from the nucleus as radius increases so there is reduced attraction
most electronegative element(s) are on the top right of the periodic table and the least electronegative element(s) are on the bottom left
relative scale / no units (0-4.00)
metals have lower ionization energies and electronegativies than non-metals
Melting points
decreases down Group 1
the elements have metallic structures which are held together by attractive forces between delocalized outer electrons and the positively charged ions (attraction decreases with distance)
delocalized electrons - electrons that move about atoms in a metallic structure
although they have the same charge down a group, there is a greater volume so the distribution of charge is less as the distance between delocalized electrons are greater
bigger ions have weaker metallic bonds
easier to melt
increases down Group 17
the elements have molecular structures which are held together by London dispersion forces (increases with number of electrons)
generally rise across a period and reaches a maximum at group 14 then falls to reach a minimum at group 18
in Period 3, the bonding changes from metallic (Na, Mg, Al) to giant covalent (Si) to weak van der Waal’s attraction between simple molecules (P4, S8, Cl2) and single atoms (Ar)
all Period 3 elements are solid at room temperature except chlorine and argon
structure explanation of trends on graph
Li, Be, B, Na, Mg, Al → metallic
C → giant covalent network
Si → giant covalent
P, S, Cl, Ar → simple molecules, single atoms
Chemical Properties
determined by electron configuration of the atom
elements in the same group contain similar chemical properties as they have the same amount of valence electrons
intermolecular forces
London Dispersion, dipole-dipole, hydrogen bonding
London Dispersion - more electrons = stronger forces = bigger atomic mass
Group 18 (noble gases)
colourless gases
monoatomic - exists as single atoms
very unreactive
inability to lose or gain electrons
do not form negative ions as the electron would be added to an empty outer energy level shell where they would experience a negligible effective nuclear force
complete valence energy levels with 8 electrons (except helium which has a complete principal first energy level with 2 electrons)
stable octet
Group 1 (alkali metals)
physical properties
good conductors of electricity and heat
due to the mobility of their outer electron
low densities
grey shiny surfaces when cut with a knife
chemical properties
very reactive
forms ionic compounds with non-metals
forms single charged ions with the stable octet of the noble gases when they react
reactivity increases down the group as the elements with higher atomic number have the lowest ionization energies
Reaction with water
reacts with water to produce hydrogen and the metal hydroxide
lithium floats and reacts slowly (releases hydrogen but keeps its shape)
sodium reacts with a vigorous release of hydrogen (heat produced is enough to melt the unreacted metal, which forms a small ball that moves around on the water surface)
potassium reacts even more vigorously to produce enough heat to ignite the hydrogen produced (produces a lilac coloured flame and moves excitedly on the water surface)
metals are called alkali metals because the resulting solution is alkaline owing to the presence of the hydrogen ion formed
reaction gets more vigorous down the group
caesium (lowest ionization energy) forms positive ions most readily
Group 17 (halogens)
exists as diatomic molecules
physical properties
coloured
shows a gradual change from gas (F2, Cl2) to liquid (Br2) and solid (I2 and At2)
chemical properties
very reactive non-metals (reactivity decreases down group)
readiness to accept electrons, illustrated by their very exothermic electron affinities
nuclei have a high effective charge, and so exert a strong pull on any electron from other atoms which then the extra electron completes the valence shell
reactivity decreases down group as atomic radius increases and attraction for outer electrons decreases
forms ionic compounds with metals and covalent compounds with non-metals
Reaction with Group 1 metals
halogens react with Group 1 metals to form ionic halides (stable octets)
example: 2Na(s) + Cl2(g) → 2NaCl(s)
the electrostatic forces between the oppositely charged ions bonds the ions together
the outer electron moves like a harpoon from sodium to chlorine and then the opposite charges of the two ions pull them together
most vigorous reaction occurs between the elements furthest apart in the Periodic Table; the most reactive alkali metal (at the bottom of Group 1) and most reactive halogen (at the top of Group 17)
Displacement reactions
the relative reactivity can be seen by placing them in direct competition for an extra electron
example: 2KBr(aq) + Cl2(aq) → 2KCl(aq) + Br2(aq)
chlorine is more reactive as it displaced bromine
stronger attraction for an electron because of smaller atomic radius
colour changes are used to determine whether or not the reaction has occured
Halides
halogens form insoluble salts with silver
adding a solution containing the halide to a solution containing silver ions produces a precipitate that is useful in identifying the halide ion
colour of the precipitate helps you identify the halide
Period 3 Oxides
Bonding of the Period 3 oxides
the transition from metallic to non-metallic character is illustrated by the bonding of the Period 3 oxides
ionic compounds are generally formed between metal and non-metal elements so the oxides of elements Na to Al have giant ionic structures
covalent compounds are formed between non-metals, so the oxides of phosphorus, sulfur, and chlorine are molecular covalent
oxide of silicon (which is a metalloid) has a giant covalent structure
ionic character of a compound depends on the difference in electronegativity between its elements
oxygen has an electronegativity of 3.4, so the ionic character of the oxides decreases from left to right, as the electronegativity values of the Period 3 elements approach this value
oxides become more ionic down a group as the electronegativity decreases
conductivity of the molten oxides gives an experimental measure of their ionic character, they only conduct electricity in liquid form if the ions are free to move (as shown on table)
maximum oxidation number of a Period 3 element is related to the group number
+1 for Group 1, +2 for Group 2, +3 for Group 13, +4 for Group 14, etc.
Acid-base character of the Period 3 oxides
acid-base properties of the oxides are closely linked to their bonding and structure
metallic elements are basic, non-metal oxides are acidic
aluminium oxide (ionic oxide with some covalent character) shows amphoteric properties
amphoteric - able to react as both an acid and a base
Basic Oxides
alkaline solutions (because of the hydroxide ions)
Na2O(s) + H2O(l) → 2NaOH(aq)
MgO(s) + H2O(l) → Mg(OH)2 (aq)
basic oxide reacting with an acid to form a salt and water
O(s) + 2H+(aq) → H2O(l)
Li2O(s) + 2HCl(aq) → 2LiCl(aq) + H2O(l)
MgO(s) + 2HCl(aq) → MgCl2(aq) + H2O(l)
Acidic Oxides
non-metallic oxides react readily with water to produce acidic solutions
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
P4O6(s) + 6H2O(l) → 4H3PO3(aq)
SO3(l) + H2O(l) → H2SO4(aq)
SO2(g) + H2O(l) → H2SO3(aq)
Cl2O7(l) + H2O(l) → 2HClO4(aq)
Cl2O(l) + H2O(l) → 2HClO(aq)
silicon dioxide does not react with water, but reacts with concentrated alkalis to form silicates
SiO2(s) + 2OH-(aq) → SiO3²-(aq) + H2O(l)
Amphoteric Oxides
behaves as a base
Al2O3(s) + 6H+ → 2Al3+(aq) + 3H2O(l)
Al2O3(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2O(l)
behaves as an acid
Al2O3(s) + 3H2O(l) + 2OH-(aq) → 2Al(OH)4-(aq)