Chemistry
1- States of matter
Matter is a word that covers every substance the universe is composed of. Matter has 2 properties:
All matter occupies space (has volume)
All matter has mass.
There are 3 states that matter can be. Solid, liquid, and gas.
Gas and liquid collectively are called fluids since they can flow.
Here are the properties of each state in a neat table.
Physical state | Volume | Density | Shape | Fluidity |
|---|---|---|---|---|
solid | fixed volume | high | has definite shape | does not flow |
liquid | fixed volume | moderate to high | no definite shape - takes the shape of the container | generally flows easily |
gas | no fixed volume - expands to fill the container | low | no definite shape - takes the shape of the container | flows easily |
Changes in state
Large changes in temperature or pressure can cause greater than expanding or contracting. They cause the substance to change its physical state. This image explains it.
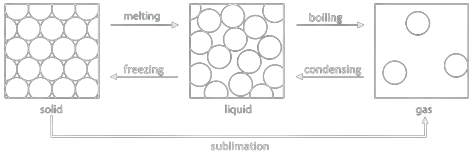
A melting point is the point in temperature at which a solid turns into a liquid.
A boiling point is the point in temperature at which a liquid turns into a gas through boiling.
Evaporation, boiling and condensation
If a liquid is left with it’s surface exposed to heat, it will evaporate. This is called evaporation, which takes place on the surface of a liquid.
At a certain temperature, gas starts forming inside the liquid and not just at the surface. Bubbles will start forming inside. This is called boiling.
The reverse of evaporation is condensation, usually brought out by cooling.
Pure substances
A pure substance consists of only one substance without any impurities.
Pure substances melt and boil at definite, specific temperatures. Impurities affect the value of the melting and boiling points of substances. An impure substance often melts or boils over a range of temperatures, not at the precise point of the pure substance.
13 - The Periodic Table
13.1 Classifying the elements
Groups and the Periodic Table
In the Periodic Table:
elements are arranged in order of increasing proton (atomic) number
vertical columns of elements with similar properties are called groups
horizontal rows are called periods.
Metals and non-metals
In the periodic table, non-metals are grouped into the top-right, above the thick line separating them from metals. The change from metallic to non-metallic properties in elements across a period is not as clear-cut as suggested by drawing the line between the two regions of the Periodic Table. The elements close to the line often show properties that lie between metals and non-metals - called metalloids. They have some of the properties of metals and others that are more characteristic of non-metals.
17 - Chemistry of our environment
17.1 Air quality
Composition of air
The atmosphere is a layer that surrounds the earth and is approximately 480km thick. It is made up of a mixture of air and water vapor, where levels of water vapor are highly variable and can range from 0.2% in areas like high mountain regions to 4.0% in areas like tropical rainforests. To compare air samples, the water is removed to produce clean dry air.
The composition of air is as follows:
78% nitrogen (N₂)
21% oxygen (O₂)
1% other gases (0.9% argon, 0.04% carbon dioxide, and other noble gases)
We need oxygen for respiration so life on earth is heavily dependent on it. We can extract from the air to treat people with respiratory problems in hospitals and for industrial welding.
Nitrogen, as it is found in the atmosphere, is unreactive. Some species of bacteria can use nitrogen directly from the air to produce amino acids, but most organisms are unable to convert gaseous nitrogen into useful products. Nitrogen is also extracted from the air and is used as an inert atmosphere for food packaging or to produce ammonia.
Carbon dioxide, although used in photosynthesis and produced by respiration, only makes up 0.04%.
Air pollution
Carbon dioxide occurs naturally in the air and is harmless in these small quantities but when present in larger quantities, it is classed as a pollutant because it is partly responsible for global warming and climate change.
Particulates are small solid particles produced during the combustion of fuels.
Sulfur dioxide
Fossil fuels often contain sulfur as an impurity. When the fuels burn, the sulfur combines with oxygen from the air to release sulfur dioxide. (SO₂).
S(s)+O₂(g)→ SO₂(g)
Sulfur dioxide is often linked to breathing difficulties due to its habit of irritating the lining of the respiratory tract and so is especially dangerous for people with asthma.
Along with nitrogen oxide, sulfur dioxide is also known to react with oxygen and water vapor in the air to form acid rain.
Acid rain is rain that has been made more acidic than normal by the presence of dissolved pollutants such as the ones previously mentioned.
It is extremely harmful to life on both land and in the water, as increased acidity levels in lakes can kill aquatic life, while many plants are extremely sensitive to pH levels. Building materials also react with acid rain leading to damage and corrosion.
The main sources of sulfur dioxide are electricity generation and burning fossil fuels such as petrol and diesel in vehicle engines.
To prevent the harmful effects of sulfur dioxide, scientists have researched ways to reduce the sulfur dioxide output. Removal of SO₂ from combustion gases is done in a chimney (‘flue’). The flue gases react with water forming an acidic solution that can be neutralized by the reaction with calcium oxide in a process known as desulfurization or ‘scrubbing’ (an acid-base reaction). To prevent SO₂ from forming, most of the sulfur containing compounds are removed before combustion, giving ultra low sulfur petrol and diesel which are required in most countries across the world.
Oxides of nitrogen
Oxides of nitrogen, also referred to as nitrogen oxides, is the general name given to represent several different oxides that include nitrogen. Oxides of nitrogen form when nitrogen and oxygen from the air react at high temperatures. An example is:
N₂ + O₂ → 2NO
Like sulfur dioxide, oxides of nitrogen have also been linked to the formation of acid rain. An example of a reaction showing how nitrogen dioxide reacts with water to form nitric acid (HNO₃) is:
3NO₂ + H₂O → 2HNO₃ + NO
Oxides of nitrogen, specifically the increase in them, are also linked to the formation of photochemical smog.
This is a form of local atmospheric pollution found in large cities in which several gases react with sunlight forming a brown haze seen over large cities.
Photochemical smog is extremely harmful to human health and has been linked to respiratory disease and asthma attacks.
Although nitrogen oxides can form naturally, most come from human activity, including vehicle emissions and power production.
One way to reduce levels of NOₓ emitted by vehicles is to use a catalytic converter. The toxic gases produced by the engine are converted into less harmful gases before they are emitted into the atmosphere.
A catalytic converter uses a rare transition metal catalyst like platinum coated as a thin layer onto a honeycomb support. Inside, carbon monoxide reacts with nitrogen oxide releasing carbon dioxide and nitrogen:
2CO + 2NO → 2CO₂ + N₂
Catalytic converters can also catalyze other reactions to further reduce the emissions of pollutant gases.
Carbon monoxide
Incomplete combustion occurs when a hydrocarbon fuel is burned in a limited supply of oxygen. One possible product is the pollutant carbon monoxide.
This should be compared to complete combustion of hydrocarbon fuels which only releases carbon dioxide and water. This occurs when there is plentiful oxygen to burn.
Carbon monoxide is toxic to humans, binding to hemoglobin and preventing them from carrying oxygen around the body.
To prevent carbon monoxide, heating systems should be regularly checked, and any inlets cleaned. Catalytic converters also prevent carbon monoxide.
Particulates
Like carbon monoxide, particulates (‘soot’) are formed as a result of incomplete combustion of fuel.
Particulates are linked to respiratory disease and there is some evidence that they can cause cancer.
An evident source of particulates is from diesel vehicles where they are produced because of a lack of oxygen getting into an engine. To prevent this, diesel vehicles are fitted with fine mesh filters that remove the particles from the exhaust gas.
17.2 Carbon dioxide, methane and climate change
Greenhouse gases
Carbon dioxide is an important pollutant. It is one of the greenhouse gases and increased amounts in the atmosphere have resulted in global warming, which causes an increase in average temperatures which leads to climate change. There are many more greenhouse gases, but we will only look at the two main ones:
Carbon dioxide
Methane
Release of carbon dioxide into the atmosphere
Carbon dioxide is produced during the complete combustion of fossil fuels.
On an similar note, methane (natural gas) produces carbon dioxide when burnt in a plentiful supply of oxygen:
methane + oxygen → carbon dioxide + water
CH₄ + 2O₂ → CO₂ + 2H₂O
Release of methane into the atmosphere
Methane is another greenhouse gas with levels increasing over recent years. Reasons include increased cattle farming (cattle emit large amounts of methane as part of their digestive system) and more waste being generated by larger populations. The decomposition of food waste under anaerobic conditions (at landfill sites) can also release large amounts of methane.
Global warming
These 2 gases occur naturally in the atmosphere and help maintain a constant temperature on earth. This relatively constant temperature is due to what we call the greenhouse effect. By trapping thermal energy reflected from earth’s surface, the greenhouse gases maintain an average surface temperature of around 15°C. Without the greenhouse effect it would be much lower, around -18°C. At this temperature, life of the type we know would not even exist. Because of human activity recently, the globe is starting to heat up. This includes things like increased fossil fuel use and changes in farming causing carbon dioxide and methane levels to rise, resulting in an increase in the average surface temperature of the Earth. This is known as global warming, and it, so far, has resulted in changing weather patterns (climate change).
Greenhouse effect
The greenhouses gases allow high-energy, short wavelength radiation from the Sun to pass through the atmosphere and reach the Earth’s surface. Some of this thermal energy is absorbed and heats the ocean and land, and some is radiated back into the atmosphere. The heat radiated back has a lower energy and a longer wavelength. The actual wavelength of this radiation falls within the infrared part of the electromagnetic spectrum. The greenhouse gases can absorb this infrared radiation and re-radiate it in all directions, with some coming back towards the Earth’s surface. This reduces the heat loss to space, increasing the temperature of the lower atmosphere. This is called the greenhouse effect because the way a greenhouse retains heat is similar to this.
Climate change
Global warming has brought a lot of consequences. They differ between countries, but general changes in weather patterns are known collectively as climate change.
The increase in average temperature has caused quicker rates of melting of the earth’s polar ice caps and glaciers, leading to rising sea levels, leading to floods in low-lying countries. It also causes changes to life cycles and migratory patterns of animals and birds. Polar bears, who rely on sea ice as they travel between different hunting grounds have also been badly affected.
This can also lead to more severe droughts. With higher temperatures, the soil dries out more quickly and this is then compounded by changes in rainfall. Low rainfall increases the chances of crop failure, and in the long term, turns land so dry it becomes a desert.
Reducing the amount of CO₂ released into the atmosphere
There are many steps that can be taken to reduce the release of greenhouse gases at an individual, national and global level. Governments around the world have recently tried to take steps together to reduce their greenhouse gas emissions. There have been many conferences. Increased public awareness and stronger presence of environmental groups has placed pressure on governments to react.
An important step towards decreased greenhouse gas emissions of carbon dioxide is to reduce our reliance on fossil fuels for transportation and electricity. Instead, we should turn to renewable sources of energy such as wind and solar.
Some countries are already doing this. Some people are switching from gas to electric cars, although there are still issues about where the electricity that powers these cars comes from.
We are also trying to remove the CO₂ from the atmosphere, by processes like photosynthesis and capturing the carbon dioxide at electricity generating plants.
Photosynthesis is a process where plants take in carbon dioxide and water, then using energy from sunlight in the presence of chlorophyll to produce glucose and oxygen.
The word equation for this is:
carbon dioxide + water → glucose + oxygen
The balanced chemical equation for this is:
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
Reducing the amount of methane released into the atmosphere
Methane is released by rotting vegetation and livestock. A good option for reducing methane emissions from cattle is to better educate people about harmful effects of excessively meat-rich diets. There is evidence that greater dependence on plant-based food is successful, with increasing numbers of vegans around the world.
Methane is also released by decomposing waste in landfill. To reduce the production of it, it is best to separate household waste from food waste so food waste does not go to landfill.
Landfill methane can also be trapped and burnt as a clean energy source for generating electricity or heat.
17.3 Water
Water is vital in our lives.
Potable water is drinking water.
Distilled water is water without any minerals, pure H₂O.
Tests for the presence and purity of water.
To test for water,
Blue cobalt chloride paper turns pink in the presence of water.
or
Anhydrous cobalt (II) chloride is converted to the hydrated version.
CoCL₂ + 6H₂O → CoCL₂•6H₂O
↑ blue ↑ ↑ pink ↑
Alternatively,
Solid white anhydrous copper (II) sulfate forms a blue, hydrated copper (II) sulfate if exposed to water
CuSO₄ + 5H₂O → CuSO₄•5H₂O
The purity of water (and any other substance) can be tested by recording its melting and boiling points. Pure water will having a melting point of 0°C and a boiling point of 100°C.
Distilled water
Tap water is a mixture of natural minerals and substances added due to human activity. Experiments should always distilled water, as the substances dissolved in the tap water can interfere with reactions.
Distillation can remove those impurities using the idea that different substances have different boiling points.
Substances in natural water
Water from natural sources can also contain substances dissolved in it. These can be beneficial or harmful.
Substances that are beneficial
Dissolved oxygen
One of the most important substances to be found in water is oxygen. The level of it determines the number and variety of organisms that can be supported.
Oxygen enters water either as a result of photosynthesis by aquatic plants of through diffusion of oxygen from the air. It is removed from the water by respiration in plants and animals.
Metallic compounds
As water passes over and through different rocks, it can dissolve metallic compounds called minerals, such as calcium and magnesium salts. There are a large range of these that are needed for good health, including group I metal ions, group II metals and a range of transition metal ions.
Substances that are harmful
Some metallic compounds
Although there are useful ones, water can be contaminated with harmful metals. Heavy metals such as lead can enter from sources like mining, metal smelting and waste disposal. Lead can cause liver and kidney damage, and mercury has been linked to nervous system damage.
Sewage
Wastewater includes a range of contaminants. It is usually carried by underground pipes (sewers) and taken to wastewater treatment plants to remove the harmful materials. Any solid can be filtered and the treated liquid returned into rivers. Leaks of sewage into drinking water can happen during natural disasters such as extreme weather. When this happens, harmful microbes enter the drinking water spreading disease such as diarrhea, cholera and dysentery.
Nitrates and phosphates
NPK fertilizers are used to increase crop yield by adding three essential plant nutrients, nitrogen, phosphorous and potassium to the soil. They are made from soluble compounds (salts) that are easily absorbed through the roots of plants. These compounds create problems if there is heavy rain after the fertilizer has been spread onto the crops. Under these conditions, instead of being taken in by the plants the fertilizer will be washed over the surface of the soil, and into waterways. This process is called run-off.
When fertilizers enter waterways, they cause rapid growth of algae, which form huge blooms that cover the surface of the water, blocking out sunlight and killing aquatic plants that live below the surface. This, in turn, kills animals in the water as it leads to a drop in oxygen levels in the water.
Nitrates and phosphates are the most common forms of pollutant that enter water because of run-off.
Plastics
Plastics are polymers and are used everyday. They are insoluble in water and so can be easily removed. Their issue is the large volume they are being released in as waste. Poor disposal of plastics combined with lack of biodegradability has resulted in polymers being released into waterways. As they do not break down they can rapidly accumulate. The impacts this can have is catastrophic.
There are several aspects to the problem of plastic on oceans:
Larger sea creatures and sea birds can be trapped by discarded fishing nets
Large scale debris such as plastic bags can be confused as prey such as jelly fish and so consumed by whales, turtles and large fish, blocking their digestive system and leading to death.
Microplastic debris accumulates in the surface, which can easily be consumed by fish and damage their digestive systems.
Chemists are designing new polymers that are biodegradable (a substance that can be broken down by microorganisms)
Purification of domestic water
Tap water undergoes several purification steps from the point it is collected to the point it is delivered. In many countries, it is taken from lakes and reservoirs.
Remove large insoluble objects such as rocks. This is known as screening. It is then taken to a sedimentation tank, where the soil and sand will drop to the bottom of the tank.
Filter the water to remove smaller insoluble particles. The water is often passed through a very fine sand to filter them out.
Use an activated carbon filter to remove dissolved organic compounds.
Disinfect the water to kill harmful waterborne microbes such as disease-causing bacteria, typically adding small amounts of chlorine to do this.
Rate of reaction
A chemical reaction can only take place when reactant particles collide with each other.
These particles must collide with enough energy to react. This is called the activation energy.
The rate of a reaction depends on the frequency and energy with which particles collide.
Changing the temperature affects both the frequency and energy of collisions.
Changing the concentration affects the frequency of collisions of dissolved reactants.
The factors that affect rate of reaction are:
Temperature
Pressure
Concentration
Surface Area
Catalysts
Collisions theory and the rate of reactions
The collision theory states that at a higher concentration and pressure, there will be more collisions that occur, therefore particles will react more frequently and faster.
Temperature
The higher the temperature, the faster the rate of a reaction.
At a higher temperature, particles have more kinetic energy, meaning the move faster and are more likely to collide with other particles. The increase in collision energy means that the number of successful collisions will increase.
Pressure
At a higher pressure, there are more collisions since the container is smaller and the gas particles become closer together. This means the particles are more likely to collide and therefore more likely to react, so a higher rate of reaction.
Concentration
The same concept used in pressure can be applied to concentration.
The higher the concentration, the more amount of particles in the same amount of space.
This means the particles are more likely to collide and therefore the rate of reaction will be higher.
Surface area
Surface area also plays a large part in rate of reactions. The higher the surface area, the more area the reactions can happen in, leading to more frequent reactions and therefore a higher rate of reaction.
Changing the surface area affects the frequency of collisions that involve a solid reaction.
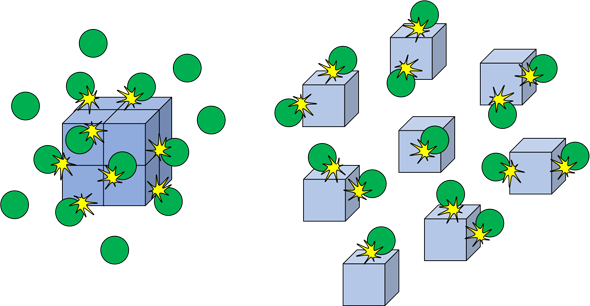
The diagram above shows an example of this. It is visible that when the cube is cut into different parts, the inside of the cube is more exposed and therefore more collisions and therefore reactions will take place.
Catalysts
The higher the amount of a catalyst, the higher the rate of reaction.
Catalysts are substances that change the rate of a reaction without being used up in the reaction, doing this by providing an alternative pathway with lower activation energy.
Since catalysts cannot lower the activation energy of a reaction, they provide a different way for the reactant particles to react with lower activation energy.
Catalysts do not produce more product, they just produce the same amount more quickly.
Measuring the rate of reaction
Two approaches can be taken in measuring the rate of reaction:
Measuring the decrease in the reactants amount.
Measuring the increase in the products amount.
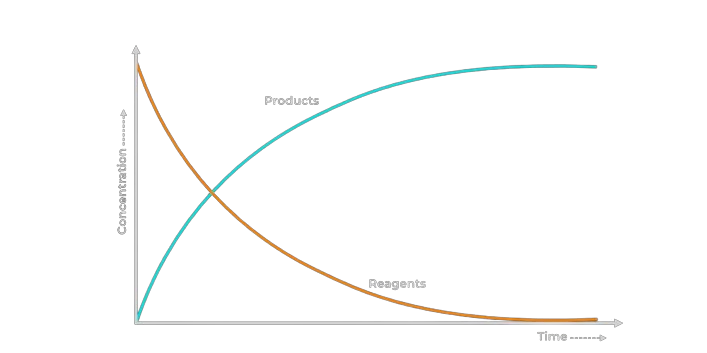
The graph above shows this the rate of change of the products and reagents (reactants) over time in a reaction. Either of these can be used to measure the rate of reaction.
The slope of the curve provides vital information about the rate of reaction.
The gradient of the graph is equal to the rate of reaction.
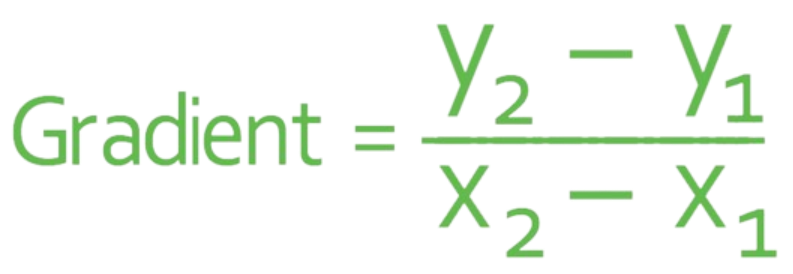
The formula for the rate of reaction:
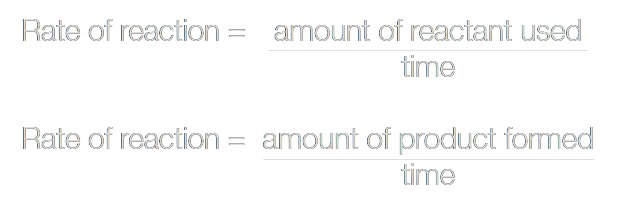
If the rate is measured from (0,0) on the graph, we call it the initial rate of reaction.
If a question asks for the rate at only a specific time, we call it the instantaneous rate of reaction.
To calculate the instantaneous rate of reaction, you must draw a tangent on the point of the graph requested and calculate the slope of that tangent.
Techniques to measure the rate of reaction practically
Gas syringe method
Inverted measuring cylinder/burette method
Mass loss method
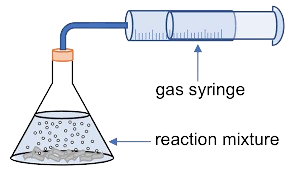
Gas syringe method
This method should be used when a gas is produced. It is especially useful with light gases and soluble gases.
A gas syringe is connected to the container.
The reaction takes place in the container.
The gas will push the gas syringe so the volume of gas will be displayed.
Record the volume of gas per unit time.
Perform the calculations necessary to find the rate of reaction.
Inverted measuring cylinder method
This method should be used when a gas is produced. It works best with insoluble gases.
The setup involves a container where the reaction will occur, an inverted measuring cylinder (connected to the first container) filled with liquid, and a third container with liquid that the measuring cylinder will be partly submerged in so that the liquid does not spill.
The reaction takes place
Gas will move from the first container to the measuring cylinder
A displacement will occur in the cylinder and the reading will be displayed on the top of the cylinder
Record the volume of gas every unit time
Perform the calculations necessary to find the rate of reaction.
Mass loss method
Used when a gas is produced. It works best with heavy gases.
The container is placed on an electrical balance.
The mass of the container with the reactants inside is measured.
The reaction takes place in the container.
The mass of the container will decrease as the gas leaves the container, so measure the mass of the container and the decrease in mass
Perform the calculations necessary to find the rate of reaction.
Disappearing X method
Used when a precipitate is formed.
Reversible reactions
Reversible reactions occur when the backwards reaction can happen relatively easily under certain conditions.
If a reversible reaction is carried out in a closed container so that the reactants and products cannot escape, a state of dynamic equilibrium can be established.
This state is dynamic because both the forward and reverse reactions are ongoing.
When this dynamic equilibrium is reached, both forward and backward reactions will occur at the same time continuously.
For a given set of conditions, the amount of reactants and products will always remain constant respectively.
La Chatelier’s principle
This principle states that when a change is made to the conditions of a system at equilibrium, the system automatically moves to oppose the change. (the three factors that can affect a system in this way are concentration, pressure and temperature.)
E.g. Increase the concentration of a substance, the equilibrium will shift to decrease the amount of substance A.
When studying the effect of a change in pressure, we consider the number of gaseous molecules only.
When increasing pressure, the equilibrium will shift to the side with less gaseous molecules.
Acids and bases
Acids taste sour, are corrosive to metals, change litmus paper to red, and become less acidic when mixed with bases.
Bases feel slippery, change litmus paper to blue, and become less basic when mixed with acids.
Acid
It is any ionic compound that releases hydrogen ions (H+) in a solution, meaning it is a proton donor.
Acids taste sour
Acids affect indicators
Acids have a pH lower than 7
Acids are proton (hydrogen ion, H+) donors
Acids react with active metals, metal H2
Acids are electrolytes
Acids neutralize bases to produce a salt and water
Bases
A base is an ionic compound that releases hydroxide ions (OH-) in a solution, meaning it is a proton acceptor.
Bases taste bitter
Affect indicators
have a pH greater than 7
they feel slippery
they are proton (hydrogen ion, H+) acceptors