Chapter 14 - Energy Conversion in the Mitochonrion
Chemiosmotic coupling takes the energy from the sun to produce a proton gradient
Mitochondria tend to associate with microtubules within a cell. There can be over 100 in each cell on average minimum. There are more mitochondria where more ATP is needed, such as in the muscles. They also provide movement energy
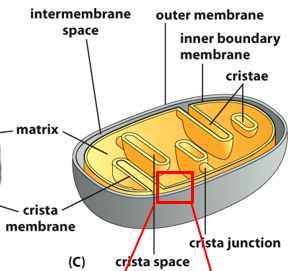
Anatomically, the mitochondria has an outer eukaryotic membrane and an inner prokaryotic membrane. This means only the outer has cholesterol, and the overall composition of lipids is slightly different. Porins are on the outside, not the inside. Cristae are foldings of the inner membrane, leading to a larger surface area for the inner membrane. This is varied from cell to cell. There is an intermembrane space between outer and inner membranes, and the outer is more permissible while the inner isn’t.
The intermembrane space has a high proton concentration, the gradient is higher outside than inside. The overall pH or cytosol and intermembrane space isn’t that different, but the inner-mitochondrial matrix has a pH of 8, with much less protons. Pumps exist to remove protons from the mitochondrial matrix
The ETC also makes NADH into NAD+, allowing it to reenter the Kreb’s cycle
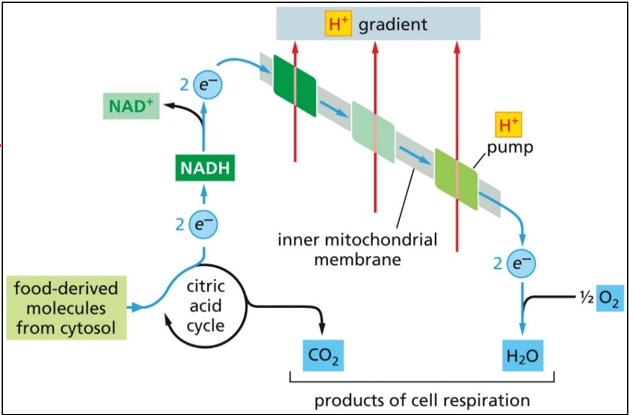
The rate of electron flow relies on the rate of ATP production, and the rate of food that is broken down. This path has no key enzyme, and only relies on oxygen supply and proton supply.
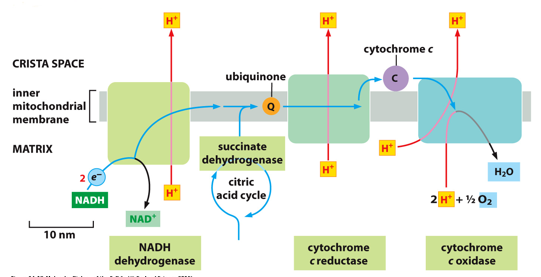
The respiration chain is the ETC, and occurs within the crista. There is one entry for electrons, which are transported to the other pumps. Each pump is Complex I, Complex III, and complex IV. Ubiquinone (CoQ) brings electrons from I to III. Cytochrome C transports electrons from III to IV. IV undergoes the redox reaction to form water. All of the reactions are redox reactions. Complex II is just a site for various electrons to enter into the ETC, also known as succinate dehydrogenase. Complex II is the only one that is NOT a pump. Memorize the names of each.
Complex I is NADH dehydrogenase, which receives dropped electrons from NADH and drops them on to ubiquinone
Complex II can receive electrons from the citric acid cycle, and put them on to CoQ
Complex III is cytochrome c reductase, which receives electrons from CoQ (ubiquinone) and drops them on to cytochrome c. It can receive from complexes I and II, which don’t interact with each other. Cytochrome C has a heme group, and it’s only function is as an energy carrier between complex III and IV.
Complex IV is cytochrome C oxidase, which receives electrons from cytochrome C and forms water (hence oxidase name). Oxygen here is the terminal electron acceptor, and this oxygen is inhaled
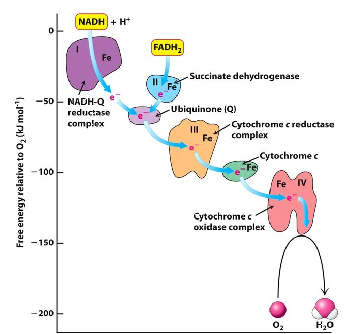
Energy is released during the ETC. Energy from NADH gets transferred to oxygen, one pair of electrons released in this process will have an equivalent of a delta G0 of -220 kJ/mol. ATP generation uses 30 kJ/mol, so this number allows for the overall coupling favored for ATP production in the mitochondrion. Many of these proteins have cofactors of Fe. Things with large reduction potentials want to donate their electrons, which is what many of these are between each step.
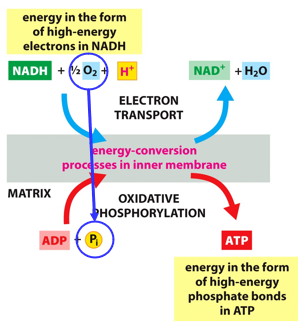
Oxidative phosphorylation will take high-energy electrons from NADH, put then through the ETC, and move that energy into ATP (from ADP and Pi). This happens in the inner membrane of the mitochondria. It is spontaneous, but very slow
There is also energy coupling in the innermitochrondrial membrane via ATP synthase. The cytosol has a pH of 7.4 and the matrix has a pH of 7.9, allowing the transport of protons out into the cytosol and down their concentration to power other reactions
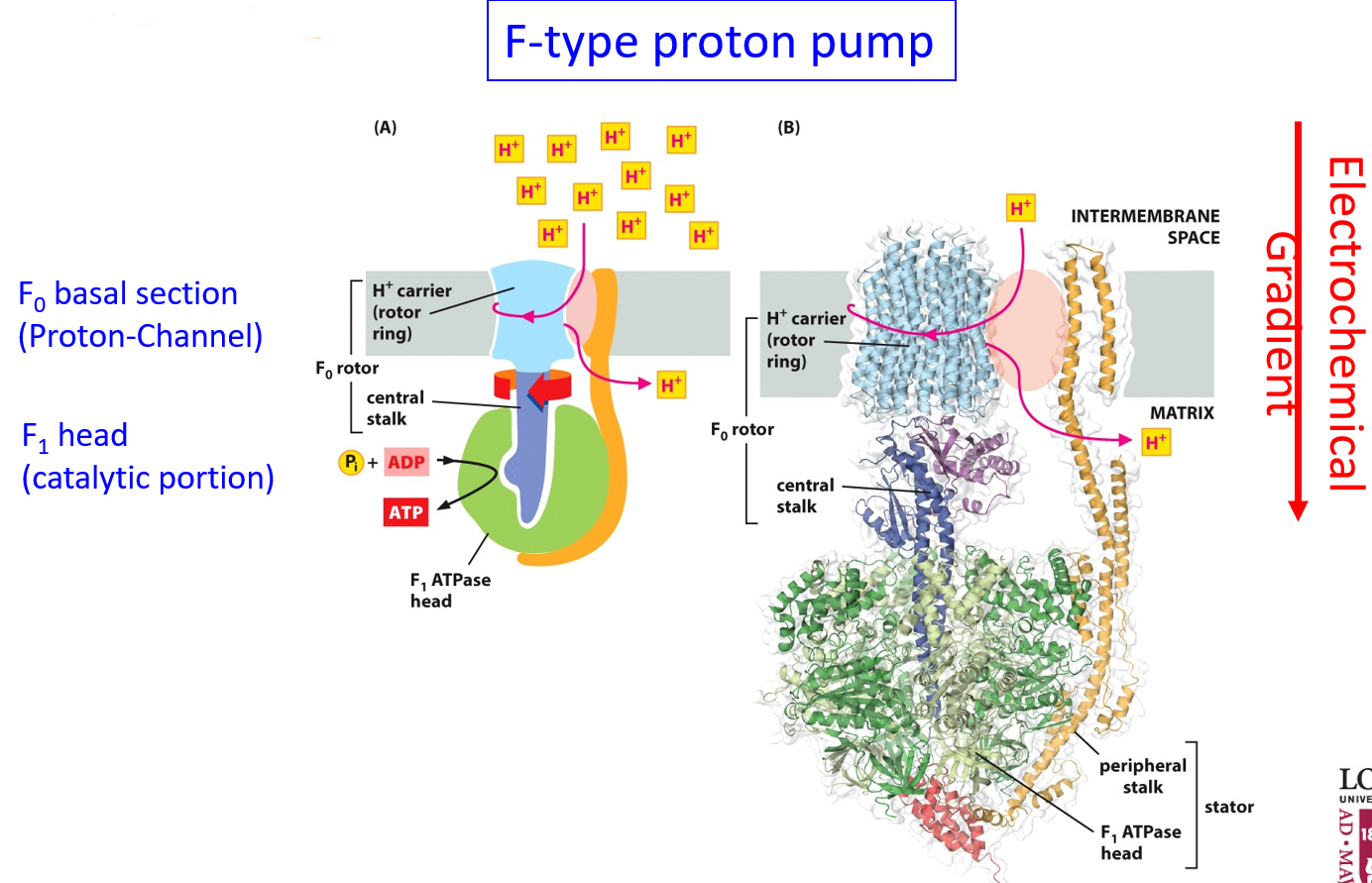
ATP synthase is an F-type pump (prokaryotic specific), consisting of an F0 basal section and an F1 head. F0 is the proton channel, and F1 is the catalytic portion (where ATP is produced). Protons enter F0, which functions as a rotor ring, which rotates the F1 central stalk. This stalk is asymmetric. The protons are also passed along after being rotated around. 10 protons can fit in a single rotation. The stalk rotates within a stationary portion, held in place by the orange portion. The proton gradient creates mechanical movement which will store the energy in ATP.
Different parts of ATP synthase include the c-ring with 10 c-subunits (for each proton), and the beta subunits which have catalytic sites. Beta subunits are the only ones that produce ATP, in sequence.
The biological function of the proton gradient is to release ATP from the active site
Rotational catalysis describes how 3 ATP molecules are produced in sequence due to the conformational change induced by the rotation of the gamma stalk. Rotation of the gamma stalk causes a conformational change in the beta subunits
The production of ATP in the mitochondrion is spontaneous, but very slow, which is what enzymes help with. The reaction is energetically unfavorable but concentrations are so high that the formation of ATP is made to be spontaneous
H+ pumps are reversible coupling devices, allowing then to switch rotations and become ATP hydrolyzers, breaking down ATP into ADP and Pi, and bringing protons into the matrix
Oxygen from breath enters the mitochondria and is used to form water during the process of the ETC, and the CO2 that is exhaled comes from the citric acid cycle
Other processes also depend on the proton gradient, such as pyruvate and phosphate import into the mitochondria. There are also voltage gated channels that allow in ADP and out ATP, usually next to ATP synthase
Uncouplers are agents that uncouple ATP synthase from the ETC, allowing protons to exit faster than ATP synthase would allow, causing them to skip ATP synthase and the overall production of ATP to halt. These kinds of proteins are found in hibernating animals and young babies (to store energy). Dinitrophenol (DNP) also does this. Instead of producing ATP, they produce heat.