Nutrients Cycle
Biogeochemical: the living distribution of chemicals.
Cycles
Influx- rate the chemical is entering.
Outflux- rate the chemical is leaving.
Reservoir- water collecting
Resident time- how long the molecules stay
Pool- smaller reservoir
Steady time- influx = outflux
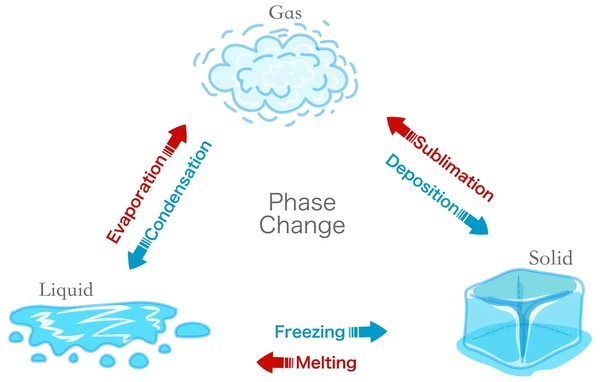
Three States of Matter
Solid, Liquid, Gas cycle
Freezing- liquid to solid
Melting- solid to liquid
Sublimination- solid to gas
Deposition- gas to solid
Evaporation- liquid to gas
Condensation- liquid to gas
Transpiration (a form of evaporation): The process by which plants lose water vapor through their leaves.
Percolation: Water moves downwards into the soil under gravitational forces.
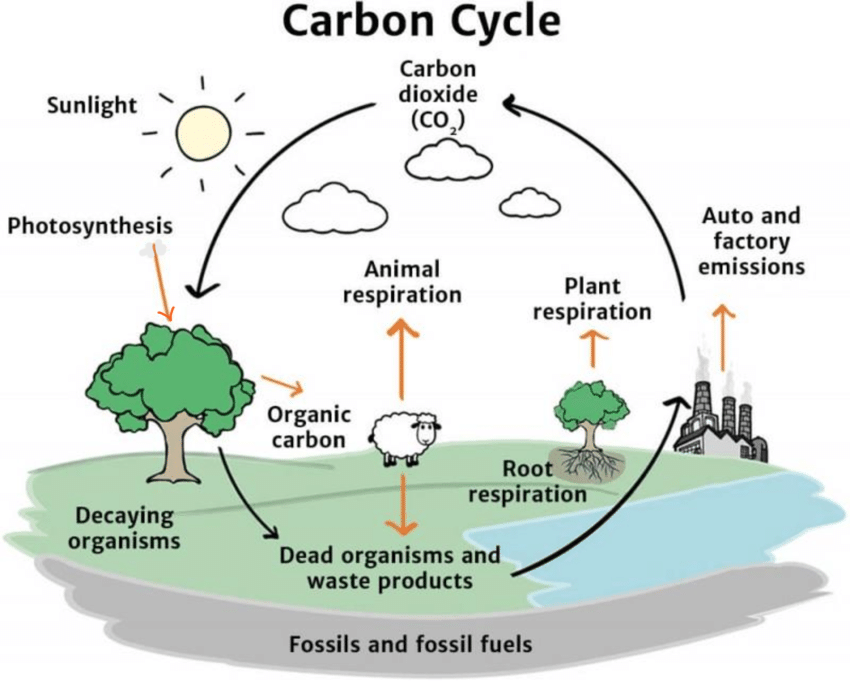
Carbon Cycle
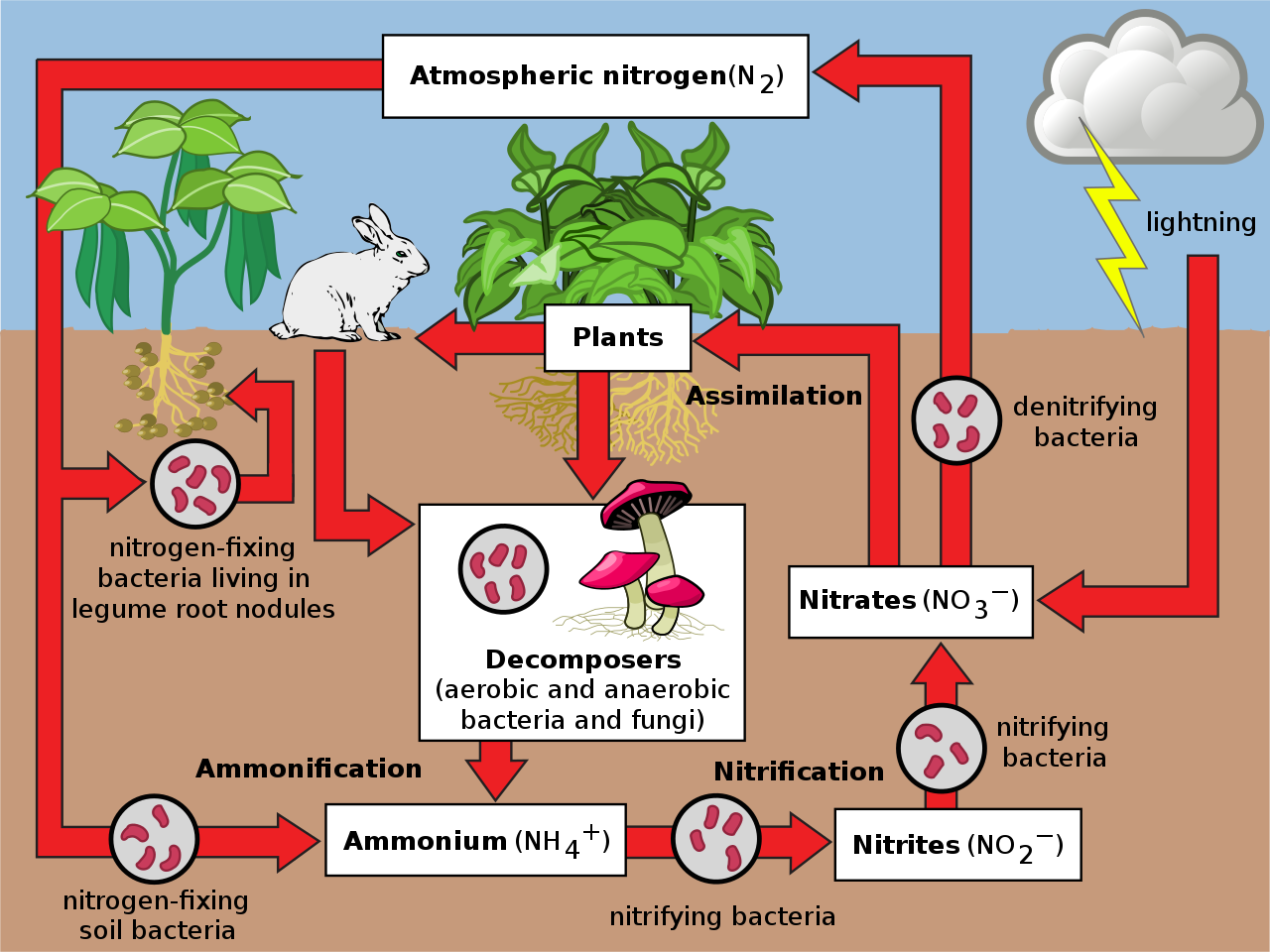
Nitrogen Cycle
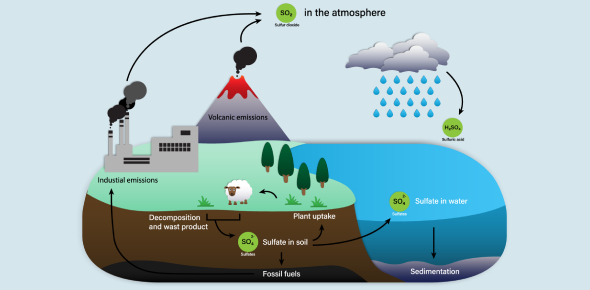
Sulfur Cycle
Photosynthesis
H2O + CO2 - sunlight = C6H12O6 (sugar) + O2
Chemosynthesis
6CO2 +6H2O + 3H2S (hydrogen sulfide) = C6H12O6 (sugar) + 3H2SO4 (sulfur compounds)
Phosphate Cycle
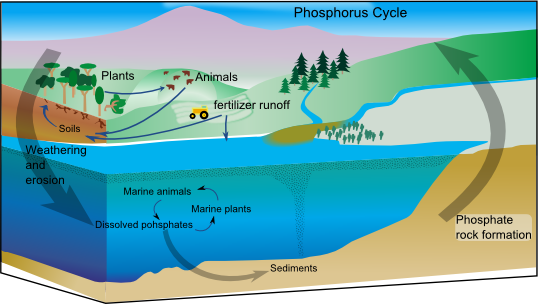
Trapped in rock- P2O2
Get it through mining
Compost for fertilizer use
Nutrient Cycle
Waste
Humans- producing more than necessary
Decomposition: How long it takes for garbage to decompose.
Non-Municpal vs. Municipal Waste
Non-municipal- Comes from factories, mining, construction sites, product production, and other industrial processes.
Municipal- Comes from households, businesses, and institutions within a community (schools).
Hazardous Waste:
Hazardous waste refers to waste materials that are potentially harmful to human health or the environment. This type of waste can be categorized into several types, each with distinct characteristics and implications for disposal and management.
Corrosive Waste:
This type of waste is highly acidic or basic, capable of causing damage to living tissue or severe corrosion of materials. Common sources include batteries, certain cleaning agents, and industrial chemicals.
Corrosive substances can lead to environmental damage if not stored or disposed of properly, potentially contaminating soil and water sources.
Ignitable Waste (Fire Hazard):
Ignitable waste can easily catch fire and sustain combustion. This category includes flammable liquids, certain solid materials, and gases. Examples include used motor oil, solvents, and certain types of paints.
Improper handling can lead to fires, explosions, and air pollution from emitted smoke and contaminants during combustion.
Reactive Waste:
Reactive waste is capable of undergoing violent chemical reactions when exposed to air, water, or other materials. This includes substances that are unstable or generate toxic gases.
Examples are explosives and certain types of batteries that can react if punctured. Proper storage and immediate attention to spills or leaks are critical for preventing hazardous reactions.
Toxic Waste (Poison):
Toxic waste contains substances that can cause harmful effects or death in humans or animals upon exposure, including heavy metals and certain chemical byproducts.
Radioactive materials, used in medical and research facilities, fall into this category and require special handling due to their long-term health risks and environmental impact.
Safe disposal methods for toxic waste often involve special facilities designed to contain and neutralize these hazards according to strict regulatory guidelines.
History of Waste Disposal
Burning
Dumping
Open landfill
Trash into a mound to decompose over time
Leachate- pollution contaminating groundwater/soil.
CH4- methane, CO2, and VOC release.
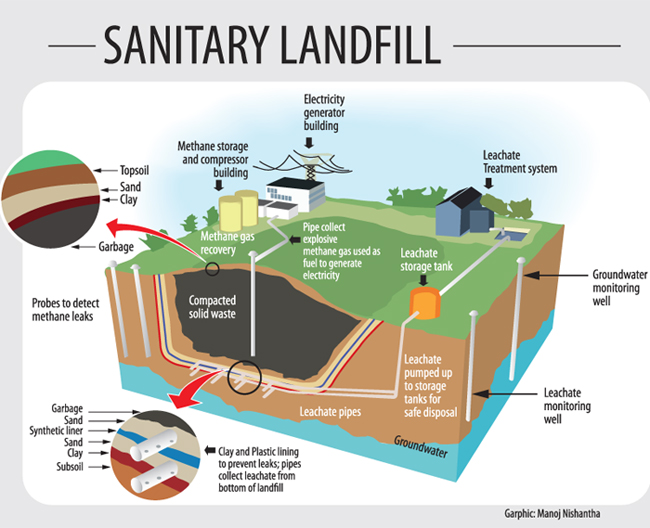
Sanitary Landfill:
Years to produce/get approved
Clay and plastic lining to prevent leachate
Monitoring wells
Pipes in waste to collect methane and used to generate electricity.
Once full- layers of soil to cover the waste. Soil prevents runoff.
Fate of trash:
Incineration- burning of trash
Compost- Composting is the process of decomposing organic matter, such as food scraps and yard waste, into a nutrient-rich soil amendment known as compost. Composting typically occurs through aerobic means, where microorganisms break down organic matter in the presence of oxygen.
Anaerobic- without oxygen
Methods to reduce waste:
refuse, reduce, reuse, recycle
Recycling:
Standard cycling- recycled becomes the same thing
Upcycle- recycle into new product
Downcycle- more common, mostly paper. recycled into less recyclable things.
Plastics:
Man-made, organisms cannot break it down.
Leach chemicals and health consequences.
Wildlife exposed to and consuming plastic.
Solutions:
alternatives
using compressed plant material
compostable plastics
eco-friendly
E-waste (electronic):
Lots produced with heavy metals
Planned obsolescence- not meant to last
Right to repair- companies design electronics so you cannot repair yourself.
Give back to the original company
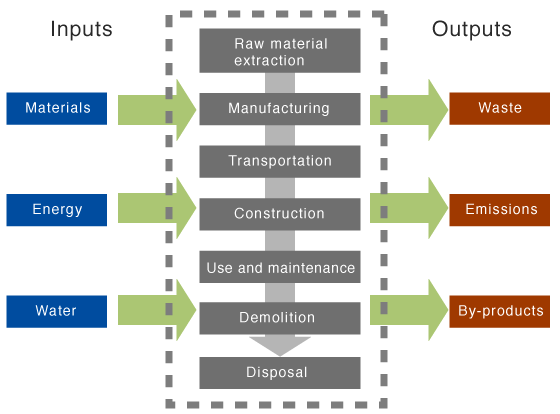
Life cycle assessments:
Compare products, compare the life cycle
Score: quantify inputs and outputs of the product cycle.
Inventory analysis- environmental impacts
Impact assessment- consider alternatives
Benefits:
environmental impacts
quantify impacts
find areas for improvement
compare products
Challenges:
how far down the chain
data availability
quantify everything
deciding which is more harmful
Example: light bulbs
What light bulbs?
incandescent, fluorescent, LED
compare- energy-efficient- lifespan- transport
 Knowt
Knowt