Alkanes and Alkenes
PART 1 - ALKANES
Alkanes are a group of saturated hydrocarbons
The term saturated means that they only have single carbon-carbon bonds, there are no double bonds |
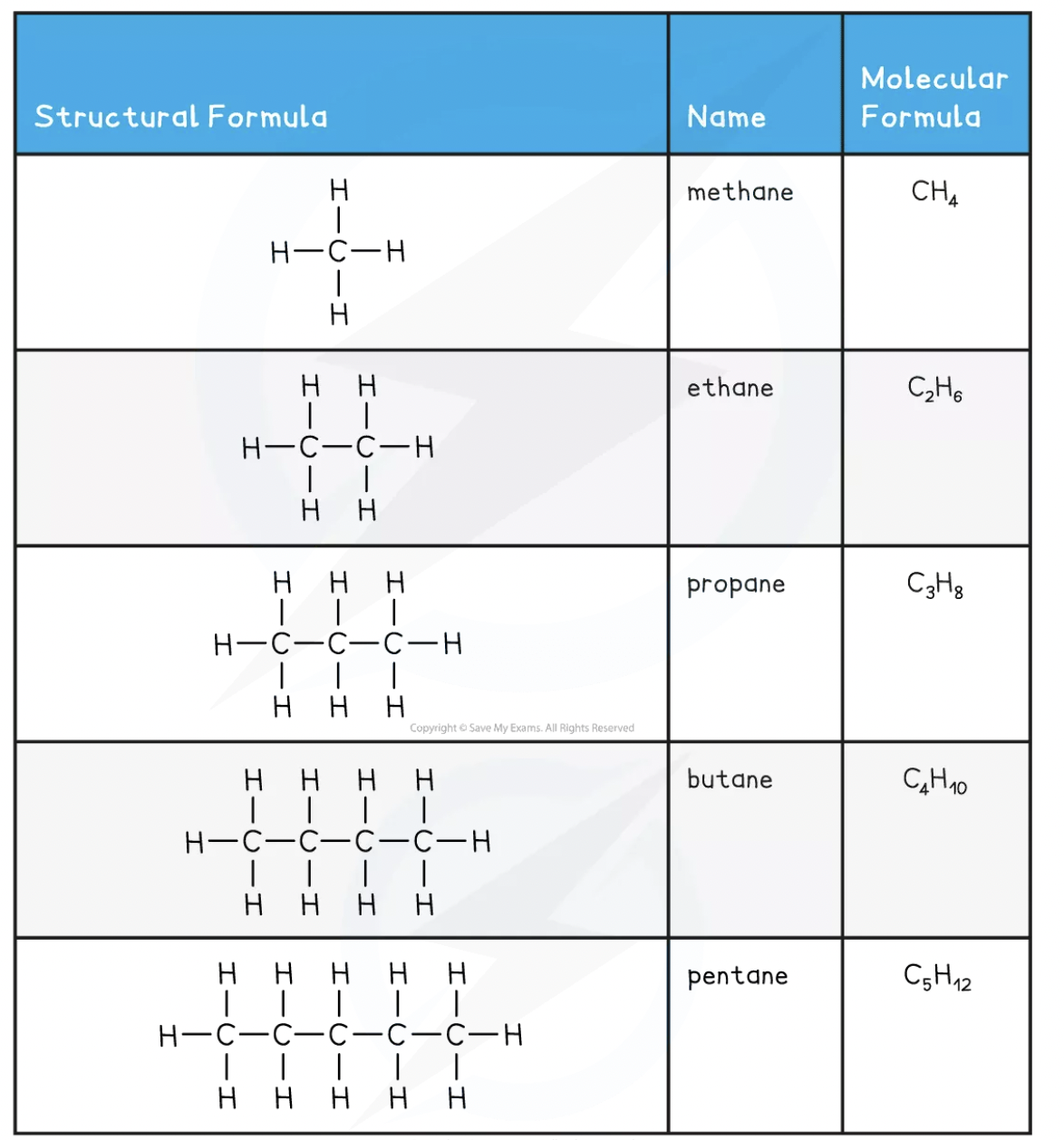
The general formula of the alkanes is CnH2n+2
This means that for every carbon they have 2 times the amount of hydrogen plus an extra 2 hydrogen (e.g. an alkane with 3 carbons would have 8 hydrogens)
They are colourless compounds that have a gradual change in their physical properties as the number of carbon atoms in the chain increases
Alkanes are generally unreactive compounds but they do undergo combustion reactions, can be cracked (we will talk more about this later) into smaller molecules and can react with halogens in the presence of light
Methane is an alkane and is the major component of natural gas
There are 5 alkanes we need to know all about. Luckily they follow a pattern that is easy to remember and everything that you need to know about these 5 alkanes is summarised in the table below!
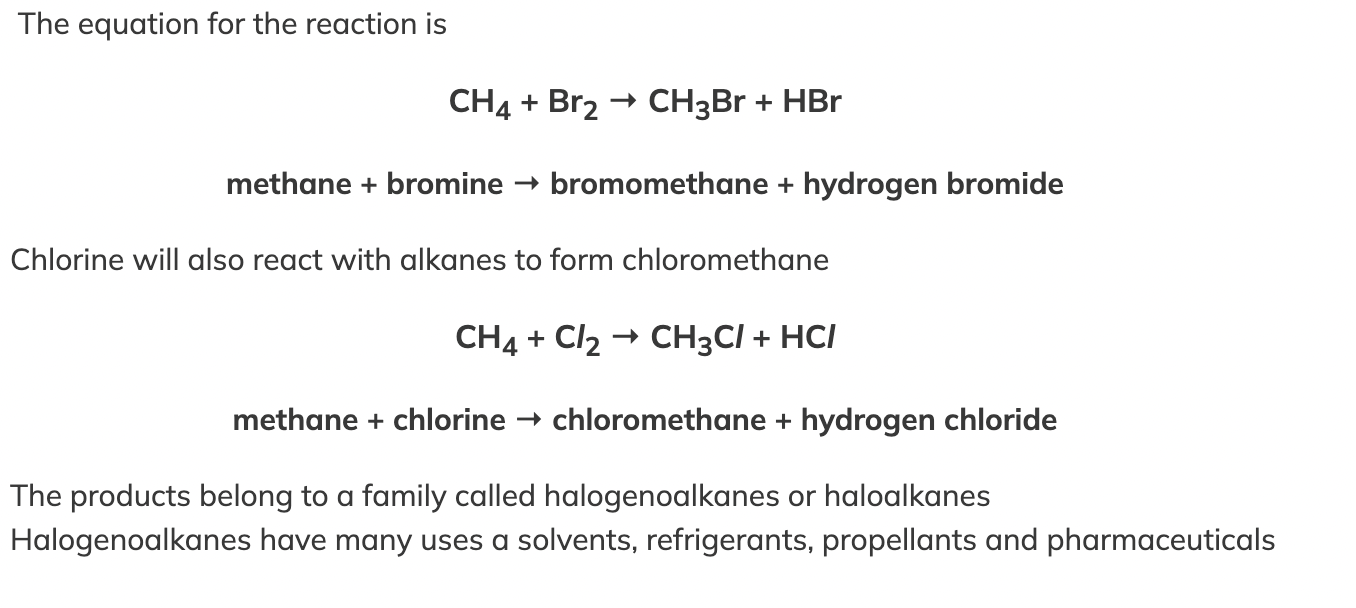
Halogens & Alkanes |
In a substitution reaction, one atom is swapped with another atom
Alkanes undergo a substitution reaction with halogens in the presence of ultraviolet radiation
PART 2 - Alkenes
The Alkenes
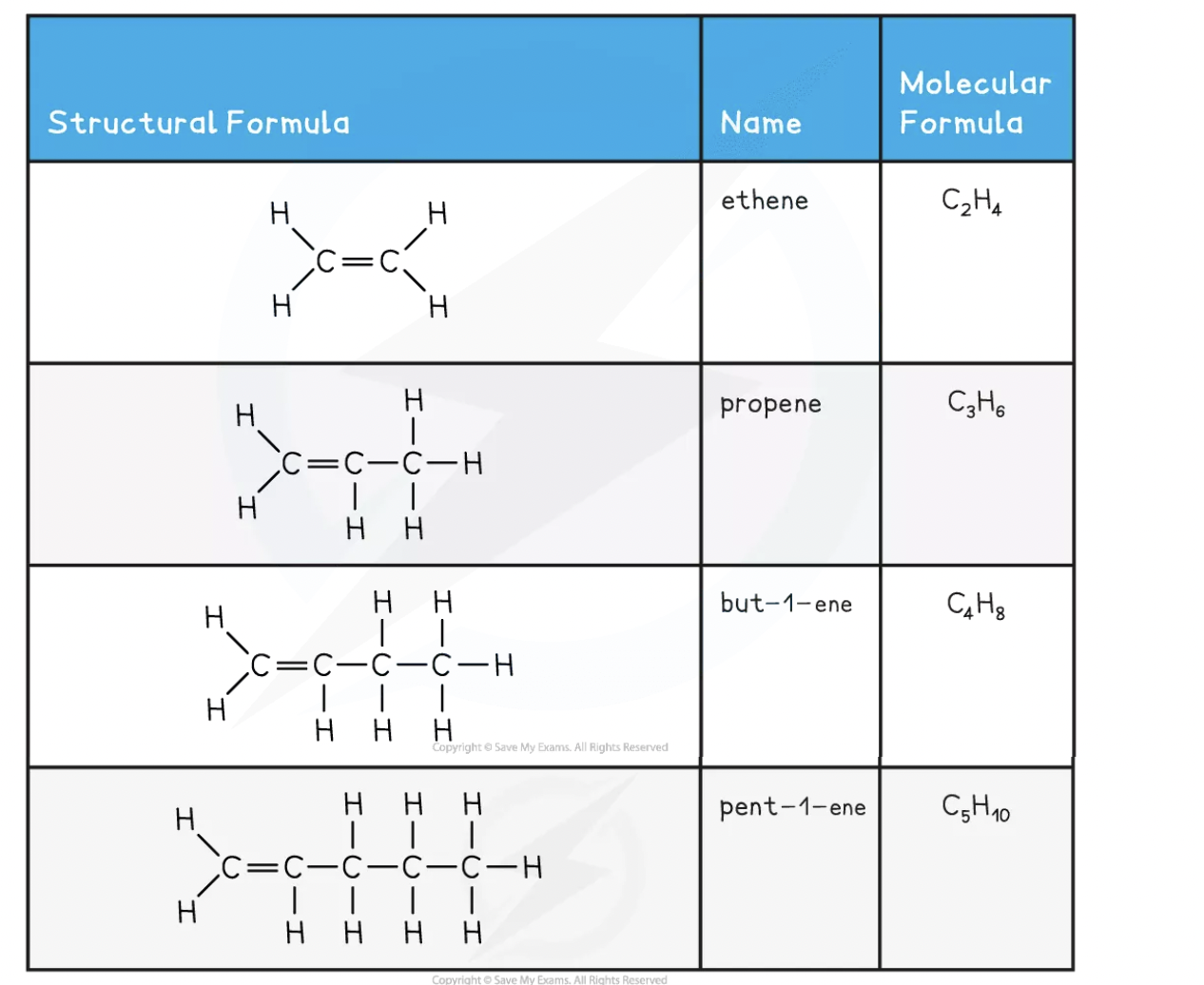
All alkenes contain a double carbon bond, which is shown as two lines between two of the carbon atoms i.e. C=C. This also means they are unsaturated
The double carbon bond is the functional group and is what allows alkenes to react in ways that alkanes cannot
The names and structure of the first four alkenes are shown below:
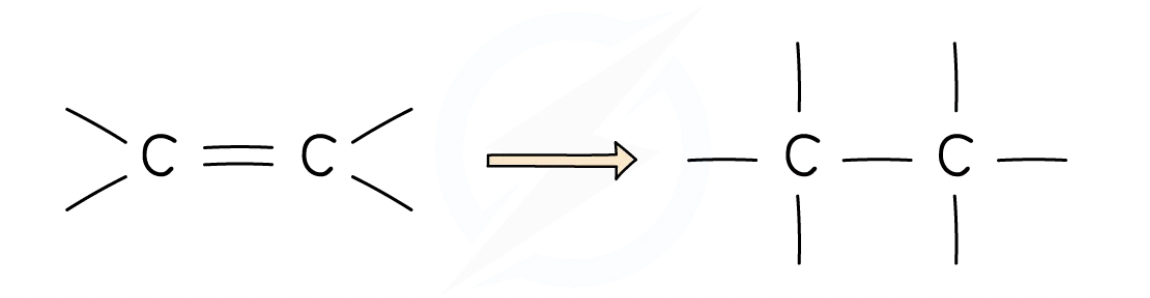
The double bond alkenes have means they can make more bonds with other atoms by opening up the C=C bond and allowing incoming atoms to form another single bond with each carbon atom of the functional group
Each of these carbon atoms now forms 4 single bonds instead of 1 double and 2 single bonds
This makes them much more reactive than alkanes
Bromine & Alkenes |
Alkenes are a homologous series of hydrocarbon compounds with at least one double bond between two of the carbon atoms on the chain
The double bond can be written as carbon to carbon double bond or as C=C
The general formula for alkenes is:
CnH2n
meaning that there are two times as many hydrogens than carbons in an alkene
Alkenes are generally more desirable than alkanes as they are more reactive due to the presence of the carbon-carbon double bond, so they can take part in reactions in which alkanes cannot, making them more useful than alkanes
They are used to make polymers and are the starting materials for the production of many other chemicals
Two useful reactions are the bromination of alkenes and polymerisation
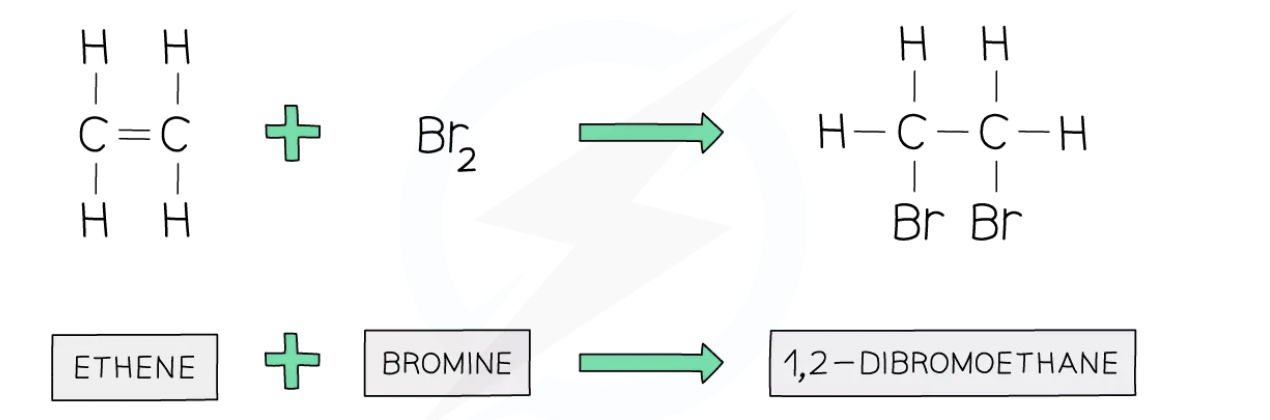
Bromination of Ethene
Alkenes undergo addition reactions in which atoms of a simple molecule add across the C=C double bond
The reaction between bromine and ethene is an example of an addition reaction
The same process works for any halogen and any alkene in which the halogen atoms always add to the carbon atoms across the C=C double bond
Bromine Water Test
Alkanes and alkenes have different molecular structures
All alkanes are saturated and alkenes are unsaturated
The presence of the C=C double bond allows alkenes to react in ways that alkanes cannot
This allows us to tell alkenes apart from alkanes using a simple chemical test called the bromine water test
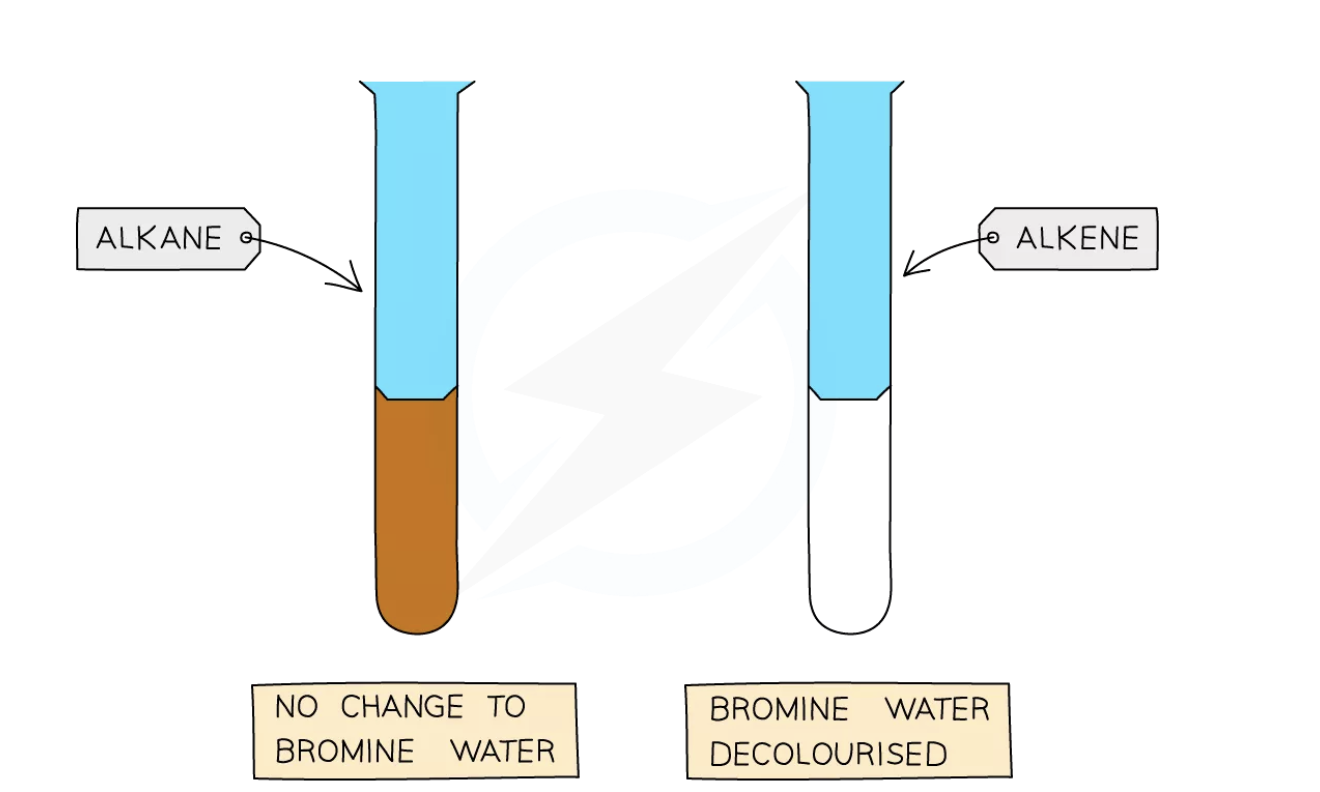
Bromine water is an orange coloured solution
When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (C=C) so the bromine remains in solution
But when bromine water is added to an alkene, the bromine atoms add across the C=C bond, hence the solution no longer contains free bromine so it loses its colour