4.properties of ether
Physical Properties
The C-O bonds in ethers are polar and thus, ethers have a net dipole moment.
The weak polarity of ethers do not appreciably affect their boiling points which are comparable to those of the alkanes of comparable molecular masses but are much lower than the boiling points of alcohols as shown in the following cases:
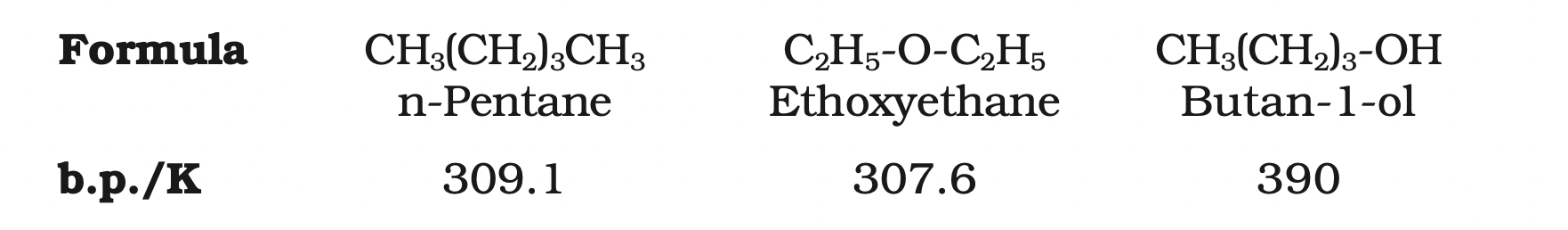
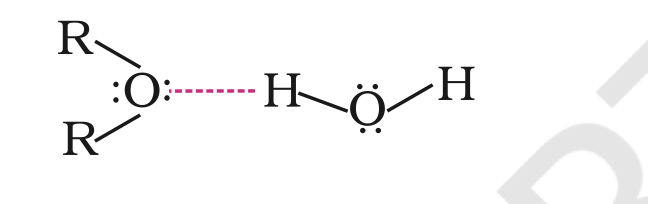
The large difference in boiling points of alcohols and ethers is due to the presence of hydrogen bonding in alcohols.
The miscibility of ethers with water resembles those of alcohols of the same molecular mass.
Both ethoxyethane and butan-1-ol are miscible to almost the same extent i.e., 7.5 and 9 g per 100 mL water, respectively while pentane is essentially immiscible with water.
Chemical Reactions
1. Cleavage of C–O bond in ethers
Ethers are the least reactive of the functional groups.
The cleavage of C-O bond in ethers takes place under drastic conditions with excess of hydrogen halides.
The reaction of dialkyl ether gives two alkyl halide molecules.
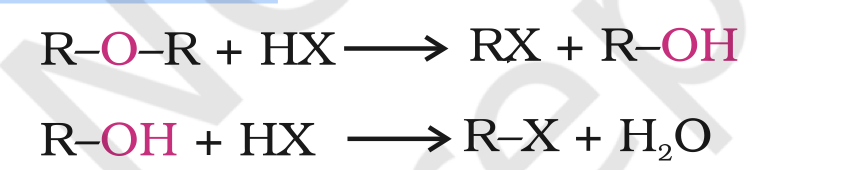
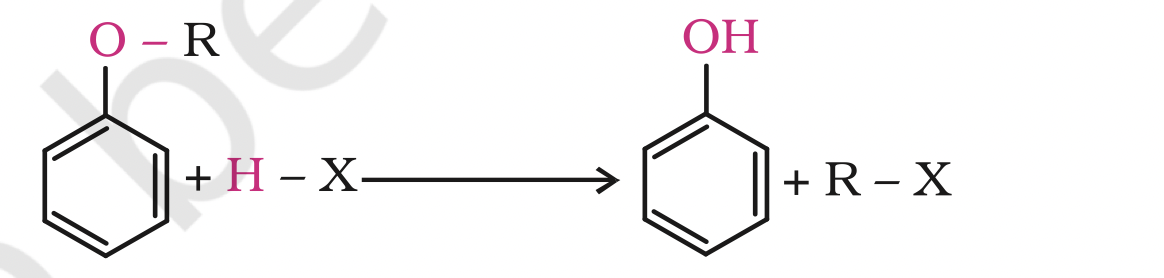
Alkyl aryl ethers are cleaved at the alkyl-oxygen bond due to the more stable aryl-oxygen bond. The reaction yields phenol and alkyl halide.
Ethers with two different alkyl groups are also cleaved in the same manner.
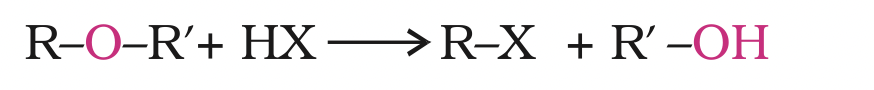
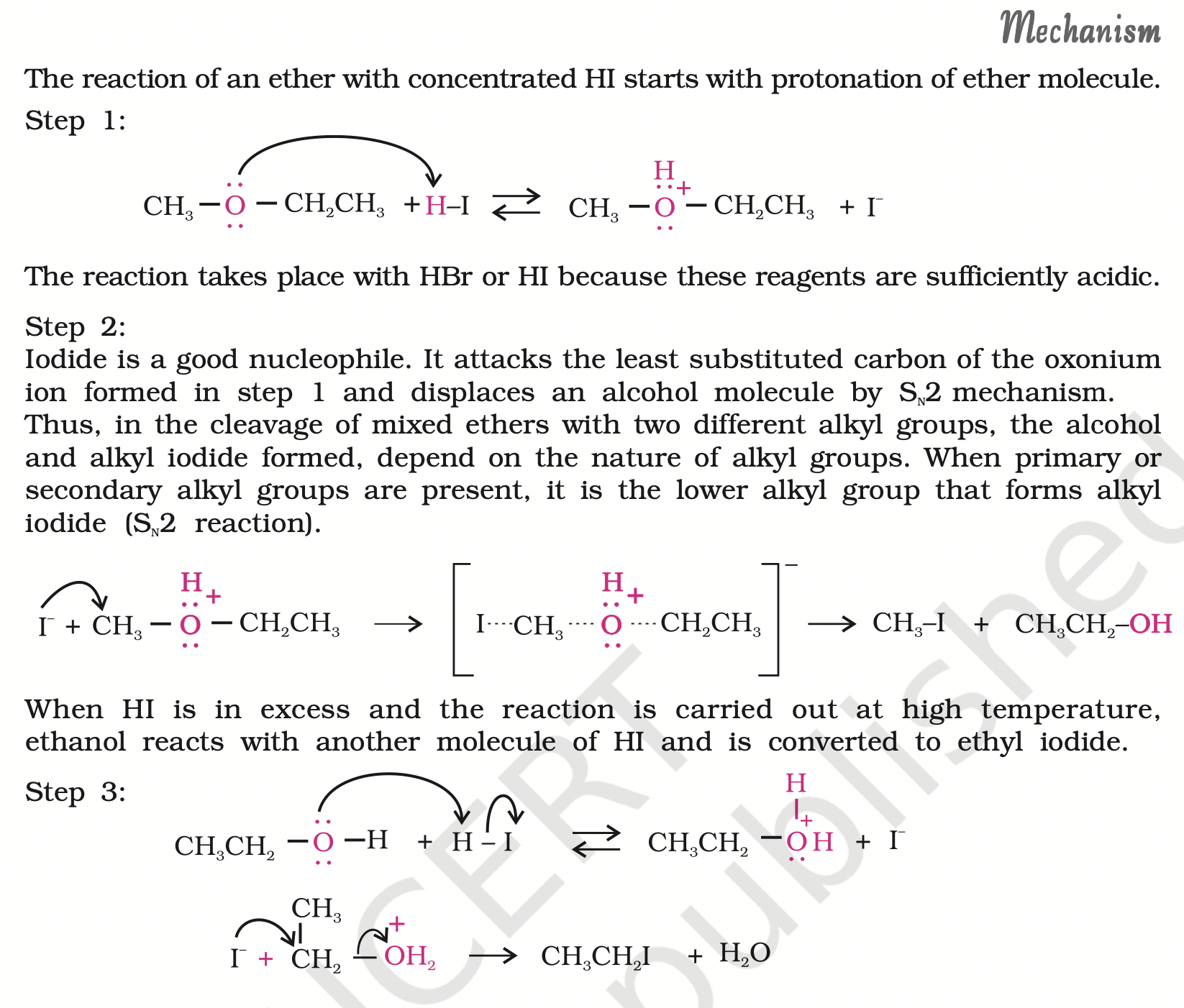
The order of reactivity of hydrogen halides is as follows: HI > HBr > HCl.
The cleavage of ethers takes place with concentrated HI or HBr at high temperature.
However, when one of the alkyl group is a tertiary group, the halide formed is a tertiary halide.
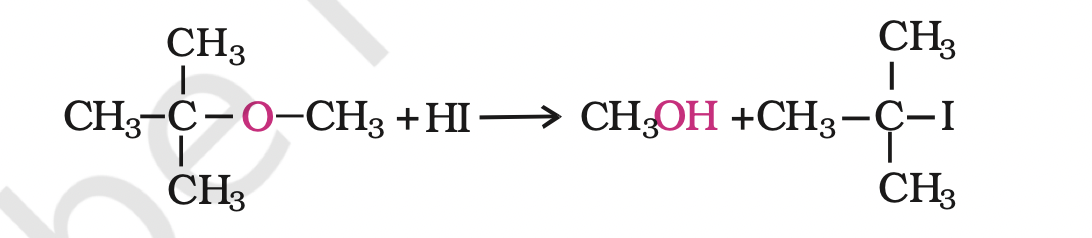
It is because in step 2 of the reaction, the departure of leaving group (HO–CH3) creates a more stable carbocation [(CH3)3C+], and the reaction follows SN1 mechanismIn case of anisole, methylphenyl oxonium ion,
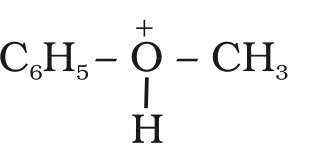
is formed by protonation of ether.
The bond between O–CH3 is weaker than the bond between O–C6H5 because the carbon of phenyl group is sp2 hybridised and there is a partial double bond character.
therfore the attack by I– ion breaks O–CH3 bond to form CH3I.
Phenols do not react further to give halides because the sp2 hybridised carbon of phenol cannot undergo nucleophilic substitution reaction needed for conversion to the halide.
2. Electrophilic substitution
The alkoxy group (-OR) is ortho, para directing and activates the aromatic ring towards electrophilic substitution in the same way as in phenol.
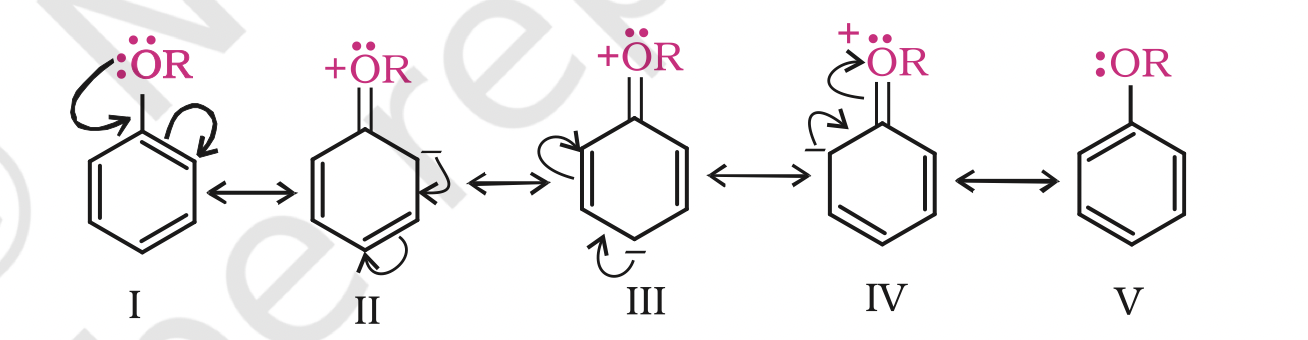
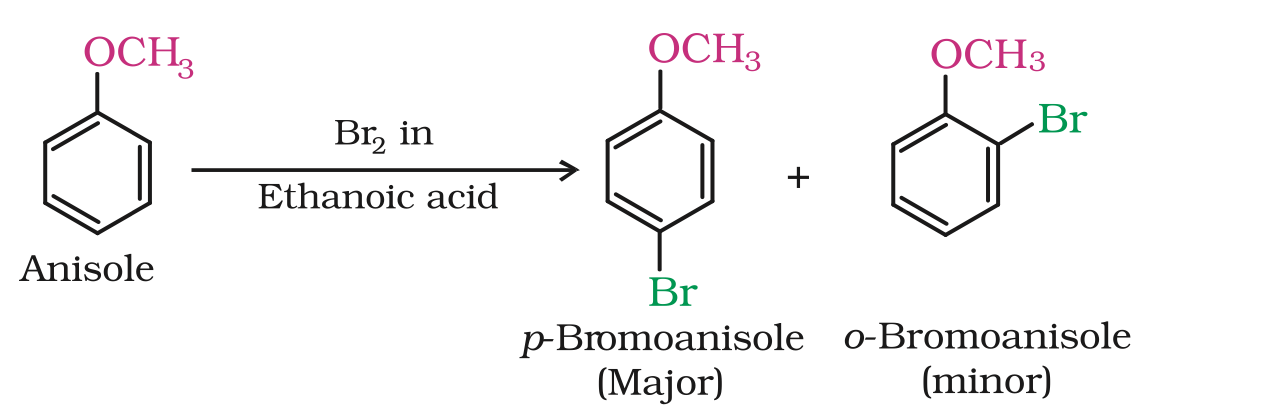
(i) Halogenation
Phenylalkyl ethers undergo usual halogenation in the benzene ring,
e.g., anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst.
It is due to the activation of benzene ring by the methoxy group.
Para isomer is obtained in 90% yield.
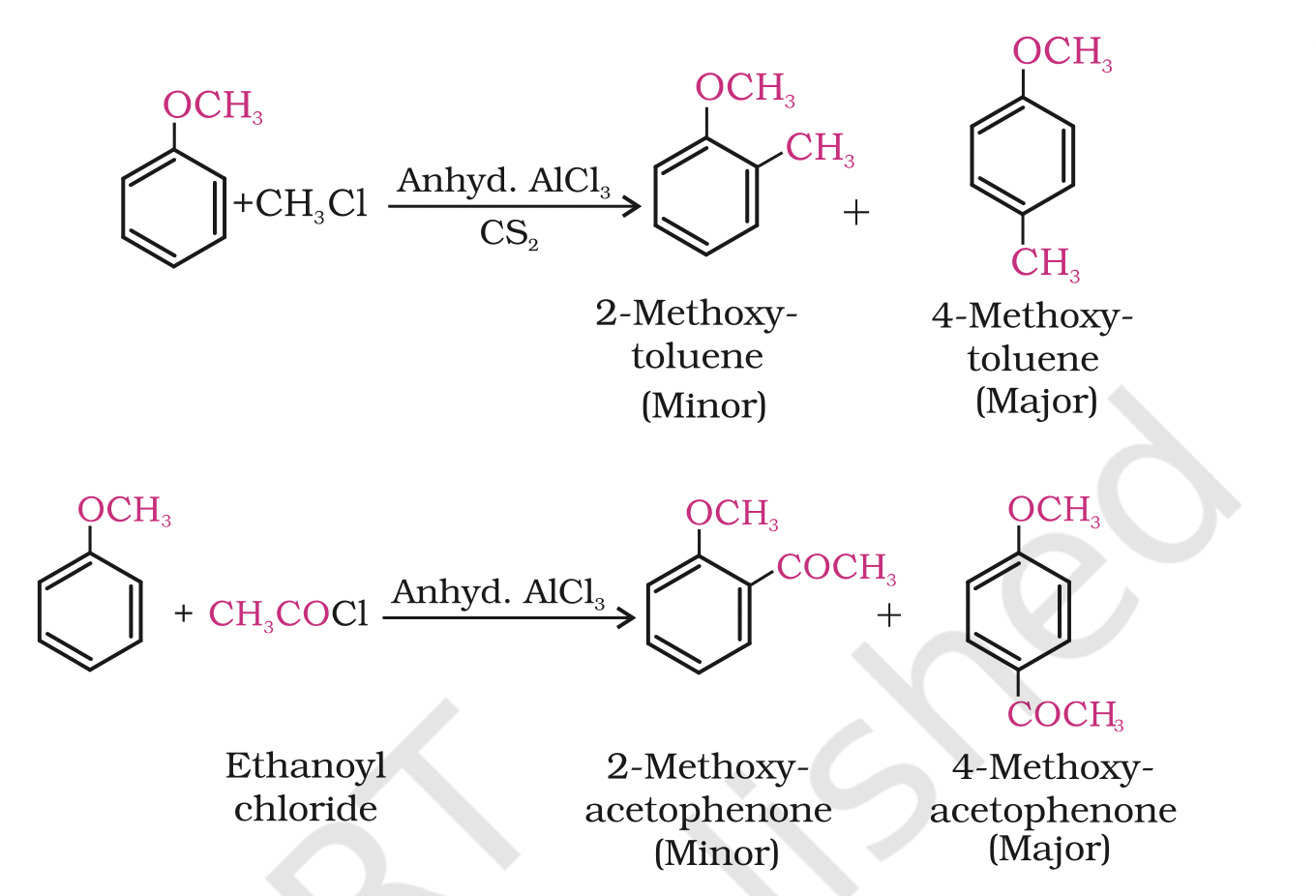
ii)Friedel-Crafts reaction:
Anisole undergoes Friedel-Crafts reaction, i.e., the alkyl and acyl groups are introduced at ortho and parapositions by reaction with alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst.
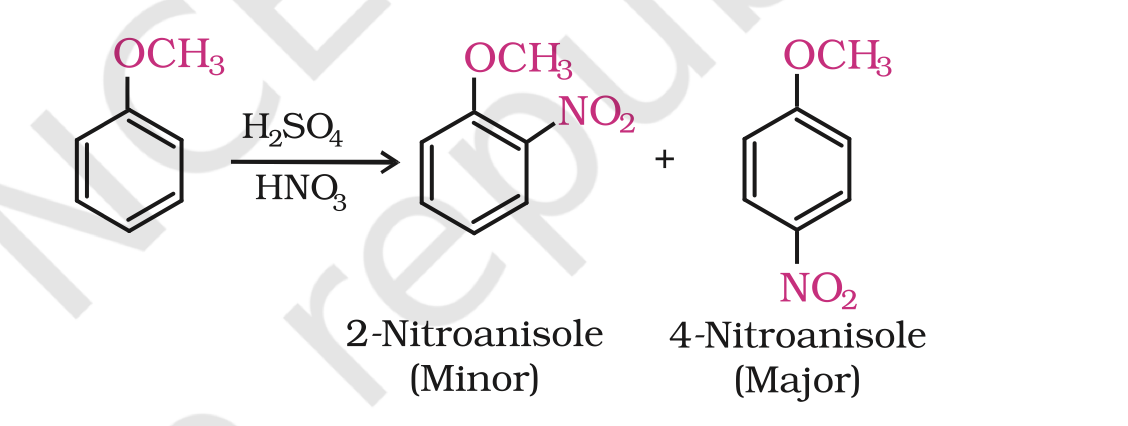
(iii) Nitration:
Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole.