Chapter 4 - The Energy of Life
All cells capture and use energy
Energy - the ability to do work/to move matter
Energy has different forms
Kinetic energy - energy of motion/movement
Potential energy - stored energy that is available to do work
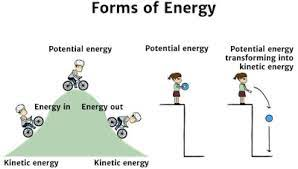 Chemical bonds are potential energy
Chemical bonds are potential energy
Molecules like glucose and triglycerides store energy in their bonds. To release the energy, the cell breaks the bonds. If the cell cannot capture the energy being released, it will be lost as heat.
Energy is converted from one form to another
Energy changed form within its biological system.
First law of thermodynamics - Energy is never created or destroyed
 Energy from the sun cannot be used directly by cells. It must be captured, stored, and converted before it is usable for cellular work.
Energy from the sun cannot be used directly by cells. It must be captured, stored, and converted before it is usable for cellular work.
Notice that heat energy is lost at each step. Heat energy is disordered, which means it cannot be used or converted back to a useful form of energy.
- It takes a lot of energy to stay “ordered”
- Cells constantly use energy to build their molecules perform chemical reactions, and carry out life’s processes
Metabolism includes all chemical reactions in cells
Chemical reactions rearrange atoms. Building complex molecules out of simple parts (like monomers) forms new chemical bonds. Breaking complex molecules into into parts (like monomers) breaks apart chemical bonds.
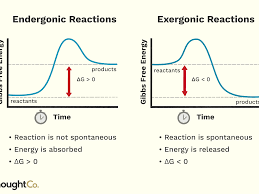
Chemical reactions can require or release energy
Endergonic - reactions that form bonds to build molecules require energy input
Exergonic - reactions that break bonds to release energy stored in the bond
 Some chemical reactions transfer electrons
Some chemical reactions transfer electrons
Most energy transformations in organisms occur in oxidation - reduction reactions.
Oxidation reactions release energy
Oxidation - is the loss of electrons from an atom or molecule, releasing energy.
The energy released by the oxidized molecule is stored in the electrons
Reduction reactions require energy
Reduction - is the gain of electrons by an atom or molecule, which requires energy.
The reduced molecule gains the energy stored in the electrons.
Oxidations and reductions occur simultaneously (“redox”)
An electron transport chain is an series of membrane proteins participating in sequential, linked oxidation - reduction reactions.
Photosynthesis and cellular respiration both use electron transport chains. As energy is released, cells store it and use it in other reactions.
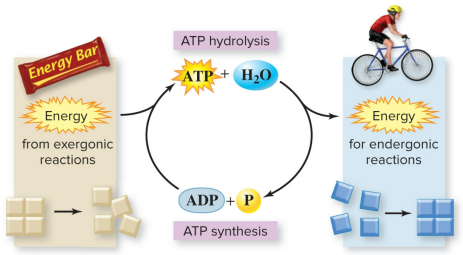
ATP is the cellular energy currency
Adenosine triphosphate (ATP) - is a nucleotide that temporarily stores energy. It is the form of energy that cells can use.
All cells rely on the potential energy stored in ATP to power chemical reactions.
ATP releases stored energy
Removing the endmost phosphate group by hydrolysis releases the potential energy stored in ATP. The cell uses this energy to do work.
ATP is formed during cellular respiration
Cellular respiration is a series of chemical reactions that release energy from sugar, producing ATP from ADP
ATP is coupled with chemical reactions
ATP breakdown is coupled with endergonic reactions.
Energy released from ATP breakdown is used to power - endergonic reactions.
ATP hydrolysis is coupled with endergonic reactions
- ATP energizes target molecule, making it more likely to bond with other molecules.
- ATP donates a phosphate group that changes the shape of the target molecule
Enzymes speed biochemical reactions
Chemical reactions in cells must occur very quickly to sustain life.
Enzymes are reusable
An enzyme - a protein that acts as a catalyst
Catalyst - speeds up a chemical reaction without being consumed, such as a protein

Enzymes speed biochemical reactions
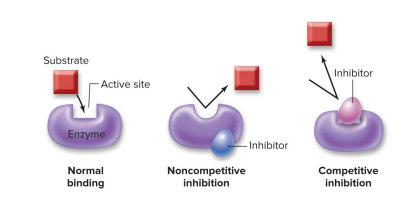
Each enzyme fits the shape of a substrate
Substrate molecules bind to the enzyme’s active site, where the chemical reaction occurs. The substrate is what the enzyme acts on.
Enzymes alter substrates to form products
Once the chemical reaction occurs, product molecules are released. The enzyme retains its original form. Products are what the reaction produces.
Enzymes speed a variety of reactions
Note that not all enzymes break a single substrate into two products. Some enzymes combine two substrates into one product, for example.
 Enzymes lower the activation energy
Enzymes lower the activation energy
Activation energy - is the energy required to start a reaction.
Without the enzyme, activation energy is high. When an enzyme binds to the substrate, activation energy is lowered.
Some enzymes require cofactors
Cofactors - Partners of enzymes that help catalyze reactions to increase enzyme activity.
- Metal ions and vitamins are common cofactors
Cells control their biochemical reactions
Only some chemical reactions are occurring in a cell at a given time. Enzyme inhibition is one way to prevent unneeded reactions from taking place.
 Many factors affect enzyme activity
Many factors affect enzyme activity
Cells control the rate of biochemical reactions is by limiting the activity of enzymes.
Inhibitors lower enzyme activity
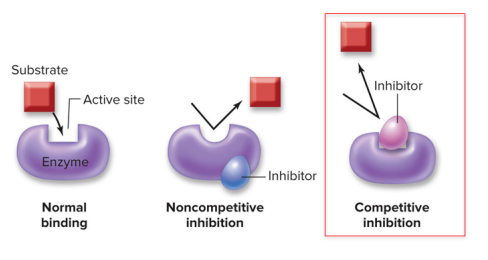
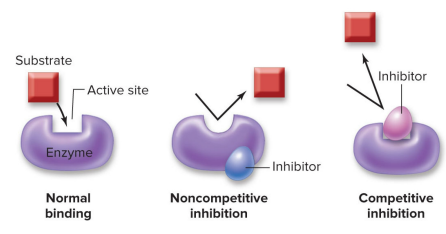
Substrate molecules typically bind to the active site of enzymes… but what if another molecule prevents it?
 Noncompetitive enzyme inhibitors change the shape of the active site
Noncompetitive enzyme inhibitors change the shape of the active site
Cells can produce molecules that bind to an enzyme outside of its active site to alter enzyme shape and prevent the substrate and enzyme from binding together.
 Competitive enzyme inhibitors block access to the active site =
Competitive enzyme inhibitors block access to the active site =
Cells can produce molecules that bind to an enzyme right at the active site, keeping the substrate and enzyme from binding together.
 Inhibitors shut down unneeded reactions
Inhibitors shut down unneeded reactions
In many cases, the inhibitor is the product of the reaction that the enzyme catalyzes. Once there is enough product in the cell, it would be a waste of energy to produce more of that product
 Substances enter and exit cells by multiple methods
Substances enter and exit cells by multiple methods
A cell’s interior is chemically different from its exterior. It maintains this difference by regulating transport of dissolved substances (solutes) across its membrane.
- Regulating what is inside of a cell is an example of homeostasis
The cell membrane forms a barrier
Only certain substances can pass through a cell membrane. Solutes enter and exit by different methods, depending on two factors:
- Concentration gradients
- The chemical nature of the substance (its polarity, charge, and size)
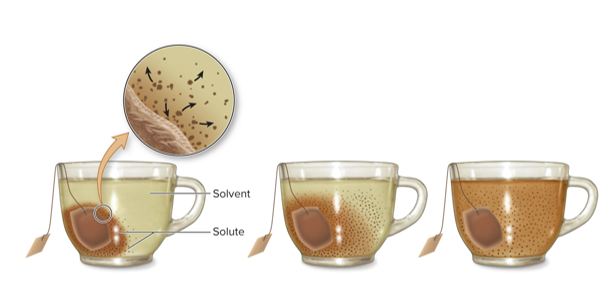
“Gradient” describes a difference between neighboring regions
 The tea is more highly concentrated near the teabag than in the rest of the cup, forming a concentration gradient.
The tea is more highly concentrated near the teabag than in the rest of the cup, forming a concentration gradient.
- Gradients are directional: This arrow points down the concentration gradient since it starts at high concentration and ends at low concentration of tea
Concentration gradients dissipate without energy input
 This is how it looks after time has passed. The concentration of tea is uniform throughout the cut (no gradient).
This is how it looks after time has passed. The concentration of tea is uniform throughout the cut (no gradient).
- The dissipation occurs by random motion
- The dissipated gradient is an example of: entropy
Maintaining a gradient requires energy
Maintaining gradients requires energy since the concentration gradient has a tendency to dissipate
 Crossing a cell membrane can occur in several ways
Crossing a cell membrane can occur in several ways
By passive transport, DOWN a concentration gradient: Simple diffusion, Osmosis, Facilitated Diffusion
By active transport, AGAINST a concentration gradient: In vesicles, by endocytosis or exocytosis
Simple diffusion does not require energy
Passive transport includes simple diffusion, which occurs when concentration gradients dissipate across a biological membrane.
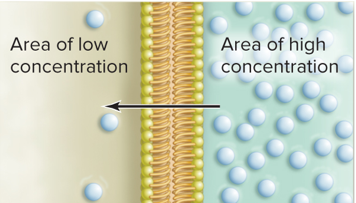 Molecules move down their concentration gradient
Molecules move down their concentration gradient
The size and chemical properties of small, nonpolar molecules allow them to pass through the membranes hydrophobic barrier. This is impossible for large polar molecules.
Osmosis does not require energy
Passive transport includes osmosis which is a simple diffusion of water across a selectively permeable membrane (down its concentration gradient)
Osmosis is driven by the concentration gradient of water
In an isotonic solution, water moves equally into and out of cells
 In a hypotonic solution, water rushed into cells from outside
In a hypotonic solution, water rushed into cells from outside
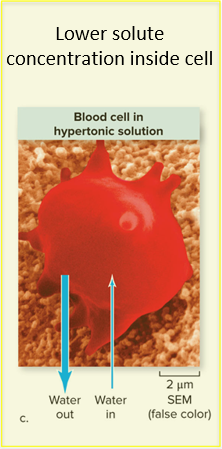
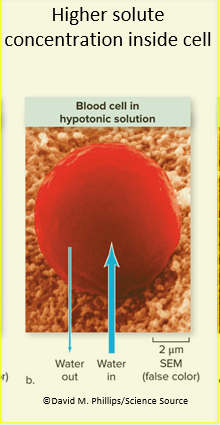 In a hypertonic solution, water rushes out of cells
In a hypertonic solution, water rushes out of cells
 Osmosis determines water content in plant cells
Osmosis determines water content in plant cells
Plants usually keep the solute concentration inside their cells lower than the outside, so that water enters the cells.
Hypertonic surroundings result in a loss of water, shrinking the large central vacuoles.
This causes cells to lose turgor pressure, causing the plant to wilt.
Facilitated diffusion does not require energy
Passive transport includes facilitated diffusion which occurs when membrane proteins transport substances across a cell membrane.
Substances move down their concentration gradient
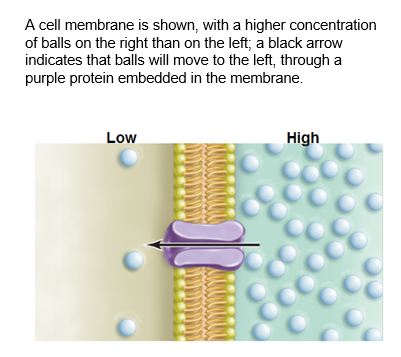 Facilitated diffusion requires membrane proteins
Facilitated diffusion requires membrane proteins
The hydrophobic tails of phospholipids in the cell membrane repel hydrophilic substances. Therefore, ions and polar molecules must pass through a protein channel in order to move across the membrane down their concentration gradient
Active transport requires energy
Active transport occurs when membrane proteins use cellular energy to transport substances across a cell membrane.
This type of membrane transport protein is often referred to as a “pump”
Substances are moved against their concentration gradient
 The sodium - potassium pump is an example of active transport
The sodium - potassium pump is an example of active transport
 Endocytosis requires energy
Endocytosis requires energy
Cells form vesicles as they engulf fluids and large molecules to bring them into the cell. Substances can be moved against of down their concentration gradients this way.
 Exocytosis requires energy
Exocytosis requires energy
Cell form vesicles to secrete large polar molecules such as proteins out of cells. Substances can be moved against or down their concentration gradients this way.
 Exocytosis brings sodium channels to the membrane
Exocytosis brings sodium channels to the membrane
When there a lot of sodium channels in a neuron cell membrane, lots of Na+ ions enter the cell.
Neurons are activated by the Na+ influx
The electrical organ produces high electrical fields.
Endocytosis removes sodium channels from the membrane
When there a few sodium channels in a neuron cell membrane, less Na+ ions can enter the cell.
Neurons activated less.
The electrical organ produces low electrical fields.
