2.4.2 - Intermolecular Forces
Introduction
Intramolecular forces - ‘primary bonds’, electrostatic attraction between atoms in a compound.
“intra” - within
“the forces that hold atoms together in a molecule”
Intermolecular forces - ‘secondary bonds’, “the forces that exist between molecules”
Also known as van der Waals forces.
Strength of intermolecular forces determine the physical properties (melting/boiling point) of a molecular (not network aka continuous covalent, since the entire structure is considered to be covalent) molecule.
Properties of Secondary Bonds
Strength of secondary bonds decreases with distance
secondary bonds between non-polar molecules diminish incredibly quickly with distance.
Dipole-dipole interactions
Dipole - molecule where the ends have opposing charges.
Dipole-dipole interactions - Attractive force between polar molecules.
(Polar molecules have permanent dipoles, because they are polar opposites)
Attraction between partially positive pole in one molecule, and partially negative pole in another molecule.
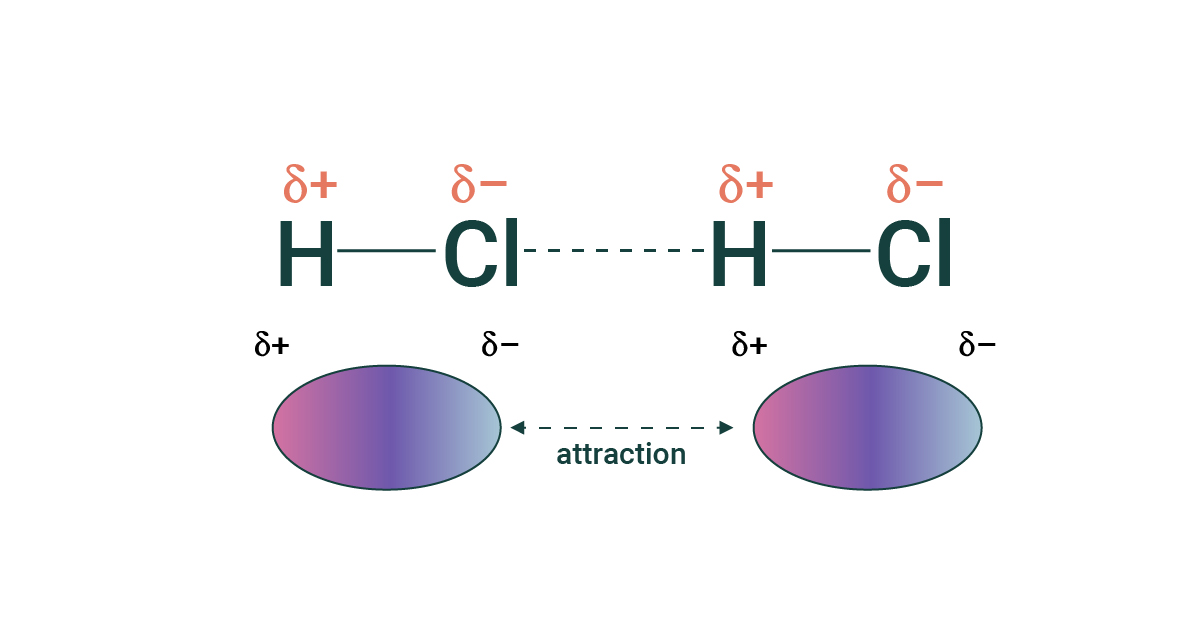
Strength of dipole-dipole interactions depend on:
Distance between molecules; the closer the distance the greater the interaction
Magnitude of electric charge:
The greater the difference of electronegativity → the greater the magnitude → the greater dipole-dipole interaction
Hydrogen Bonds
Hydrogen bonds - A type of dipole-dipole interaction that occurs between: a partially positive (𝛿+) hydrogen and a partially negative (𝛿-) oxygen/nitrogen/fluorine.
Oxygen/nitrogen/fluorine = most electronegative elements, therefore making hydrogen bonds a quite strong form of dipole-dipole interactions.
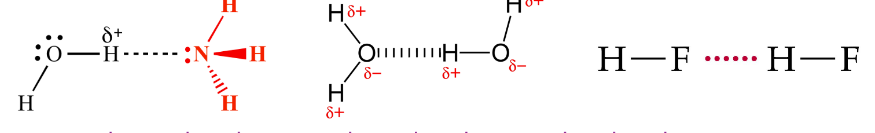
Dispersion forces
Dispersion forces - Weak forces of attraction between both polar and nonpolar molecules.
Because of random electron movement, even nonpolar molecules can have temporary dipoles
Random movement of electrons causes temporary dipoles - asymmetrical distribution
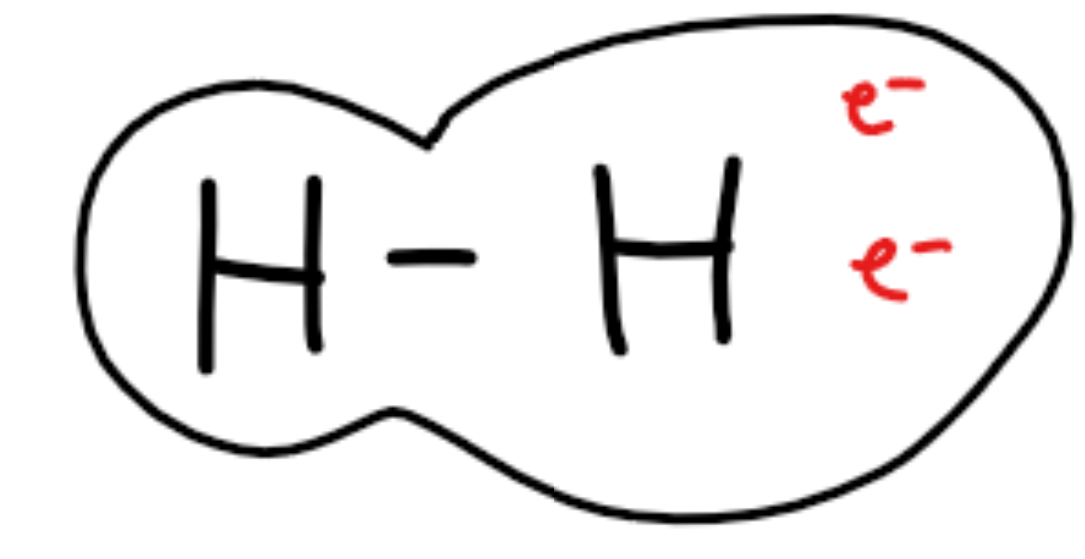
Temporary dipoles form temporary attractions.
Induced Dipoles
Induced dipoles - formed from when one molecule is temporarily polarised, as with dispersion forces (process is called induction)
This results in another molecule having its symmetrically-distributed electrons repelled by the extensive partial negative charge of the first molecule.
Electrons of second molecule are forced to one side, causing a partial negative on the opposite side of the first molecule’s partial positive.
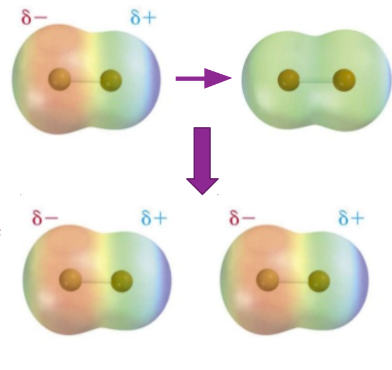
Induction is more successful with larger atoms and molecules - the negatively charged electrons are further away from the positively charged nucleus and thus are easier to shift.
Increased size - greater number of electrons; increases size of potential temporary polarisation of molecule; leads to greater induced dipole-dipole interactions.
Ion-Dipole Interactions
Ion-Dipole interactions - Occurs between ion and a polar molecule.
Used to determine solubility of ionic compounds.
Strongest type of secondary force.
Strength of Secondary Bonds
Strength | Bond type | Description |
|---|---|---|
Strongest | Ion-Dipole | Ions with polar molecules |
Hydrogen | Hydrogen with oxygen/nitrogen/fluorine | |
Dipole-dipole | All polar molecules | |
Weakest | Dispersion | All polar and nonpolar molecules |
Melting/Boiling Points
Nonpolar molecules have greater boiling points as their size increases.
Polar MP/BP vary widely.
Depending on structure of molecule, both hydrogen and dipole-dipole bonds can be involved.
Hydrogen bonds → higher boiling point because hydrogen bonds are the second strongest type of intermolecular force.