Workshop 2
Question 1: Which techniques would we use to assess the amount of crystalline material in a PET object?
DSC (Differential Scanning Calorimetry): Quantitative through ΔH (accurate).
WAXS (Wide-Angle X-ray Scattering): Gives absolute crystallinity value, but may have inaccuracies due to background.
Density measurements: Very accurate.
Question 2: Crystal growth rate G of polymers and its temperature dependency.
a) Factors leading to the bell-shaped dependency:
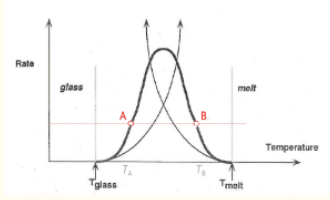
Nucleation vs. Diffusion: At low T, nucleation is high but diffusion is low; at high T, diffusion is high but nucleation is low. The maximum growth rate occurs where both processes are balanced.
b) Microstructures at TA and TB:
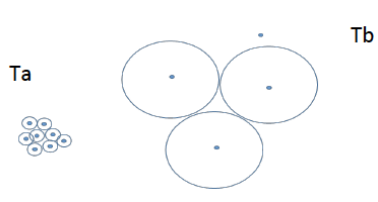
At TA: Many nuclei lead to small crystals/spherulites due to low diffusion.
At TB: Few nuclei lead to large crystals due to high diffusion.
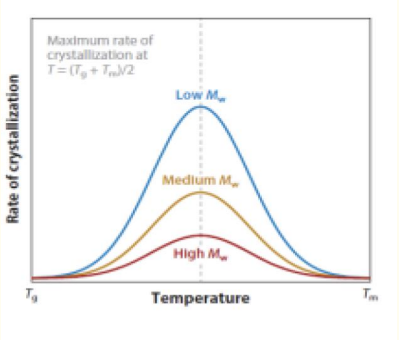
c) Crystal growth rate for different molecular weights (Mw):
Higher Mw makes crystallization harder due to more entanglements, reducing the growth rate.
Question 3: Why does the glass transition temperature (Tg) of polyamides depend on air humidity?
Water acts as a plasticizer, interacting with hydrogen bonds in polyamides, which decreases Tg.
Question 4: DSC plots for PET films.
a) Quenched PET film: Shows a glass transition (Tg), cold crystallization, and melting.
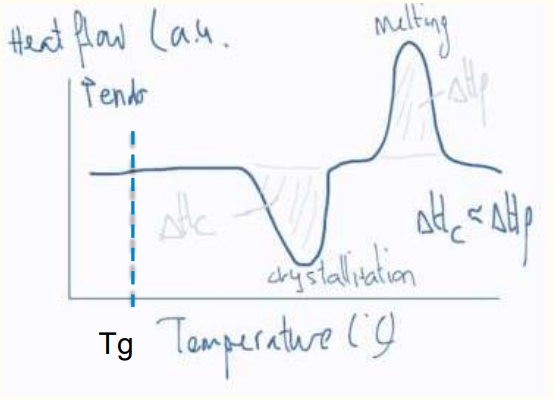
b) Annealed PET film: Shows reduced cold crystallization and increased melting due to pre-existing crystallinity.
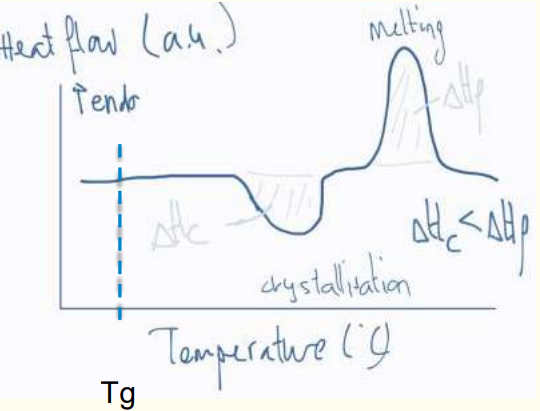
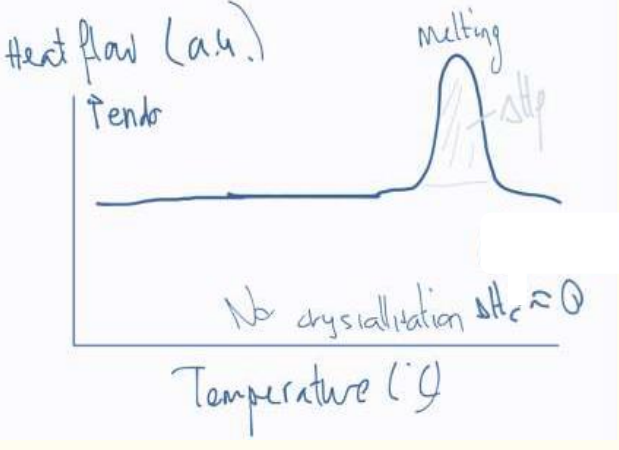
c) Fully crystallized PET film: No cold crystallization peak; only melting is observed.
Question 5: Factors affecting Tg.
Chain flexibility & molecular structure.
Branching and cross-linking.
Polymer molar mass (MM) / chain length.
Chemical composition.
Additives.
Question 6: Processing precautions to minimize amorphous components during solidification.
Slow solidification rate and high crystallization temperature.
Post-deposition procedures like thermal annealing.
Heterogeneous nucleation (e.g., nucleating agents, epitaxy).
Crystallization from dilute solutions to reduce entanglements.
Stress and pressure.
Question 7: Temperature range and type of polymer for toughness.
Use semicrystalline polymers above Tg for toughness. Below Tg, polymers are brittle; above Tg, they exhibit plastic deformation.
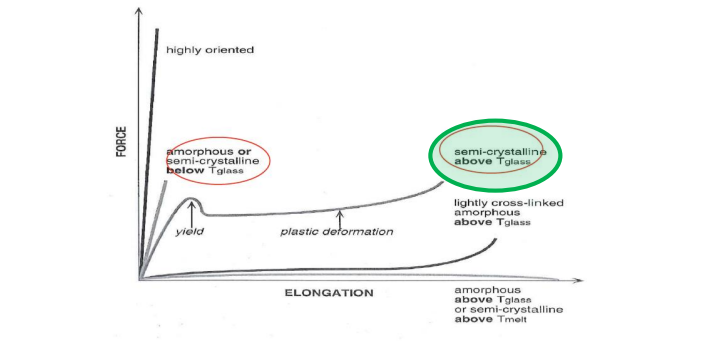
Question 8: Importance of Tg and Tm in structural applications.
Tg and Tm determine the application temperature range.
Semicrystalline polymers are tough between Tg and Tm.
Amorphous polymers can be used as elastomers above Tg.
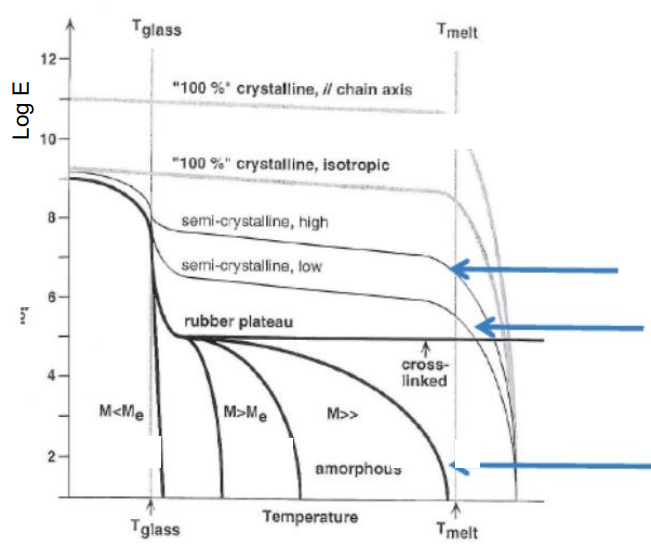
Question 9: Modifying Young’s Modulus of an elastomeric polymer.
Increase cross-linking.
Increase molecular weight (MM).
Question 10: Crystallinity in conductive polymers.
A) Properties maximized: Carrier mobilities.
B) How to increase crystallinity:
Use high molecular weight (MM).
Use small molecules to improve electrical conductivity.
Use nucleating agents and epitaxial crystallization.
Use high-boiling point solvents for slow crystallization.
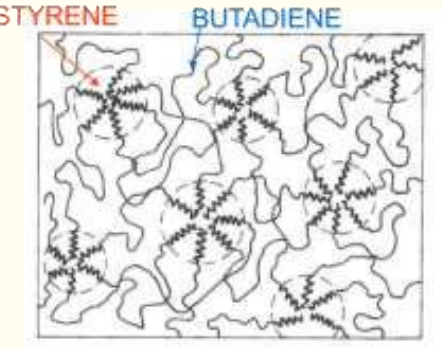
Question 11: Block-copolymers of PS and PB.
i) Why PS and PB are selected: PS is brittle, PB is an elastomer with low Tg. Combining them optimizes mechanical properties.
ii) Block copolymers vs. random copolymers: Block copolymers create physically crosslinked structures (e.g., rubbery PB domains crosslinked by PS), offering better mechanical properties than random copolymers.
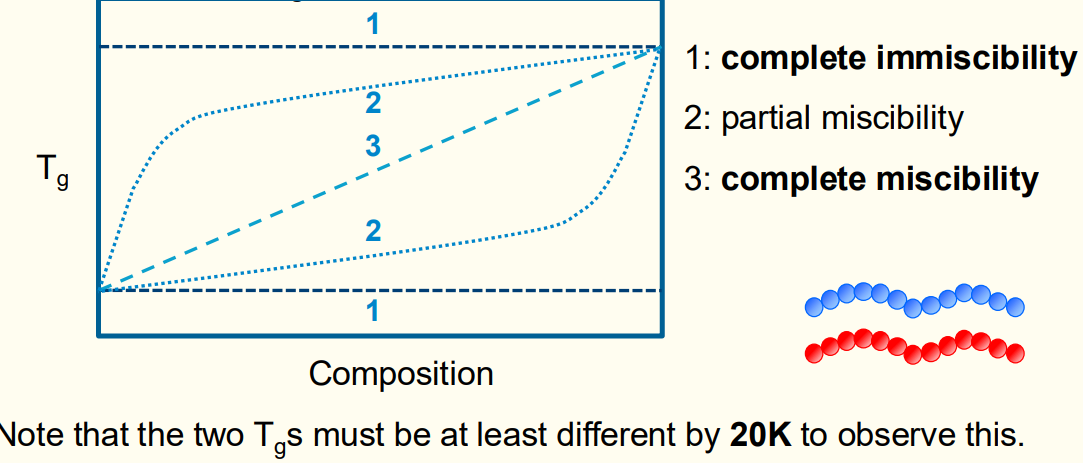
Question 12: Increasing Tg of a polymer.
Cross-linking.
Making a miscible blend with a high Tg polymer.
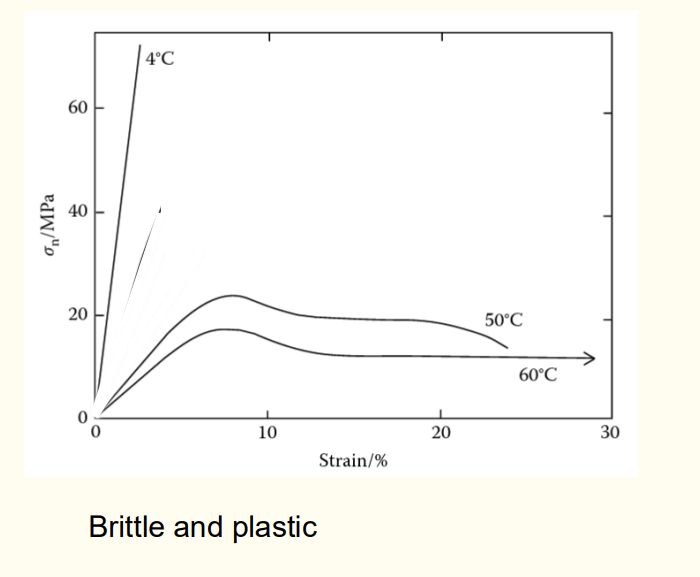
Question 13: Stress-strain curve of PMMA below and above Tg.
Below Tg, PMMA is brittle. Above Tg, it exhibits plastic deformation, with higher temperatures leading to more ductile behaviour.
Question 14: Di-block copolymer of Nylon 6 and Nylon 10.
The block copolymer will display two melting temperatures due to microphase separation (Strong Segregation Limit, χABN>>10).
The block copolymer will have a higher degree of crystallinity compared to a random copolymer because the blocks can phase separate and crystallize independently.
Question 15: DSC thermograms with and without nucleating agents.
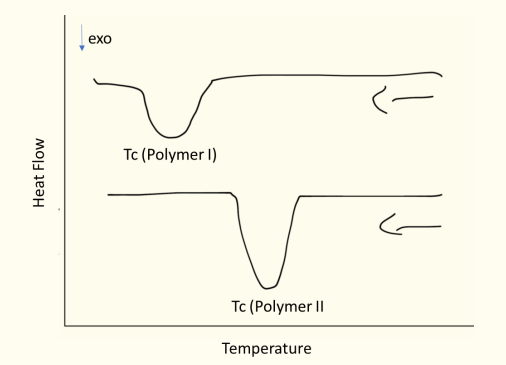
Sample II contains the nucleating agent, as it crystallizes at higher temperatures, indicating assisted nucleation.
Question 16: Behaviour of amorphous polymer chains under stress.
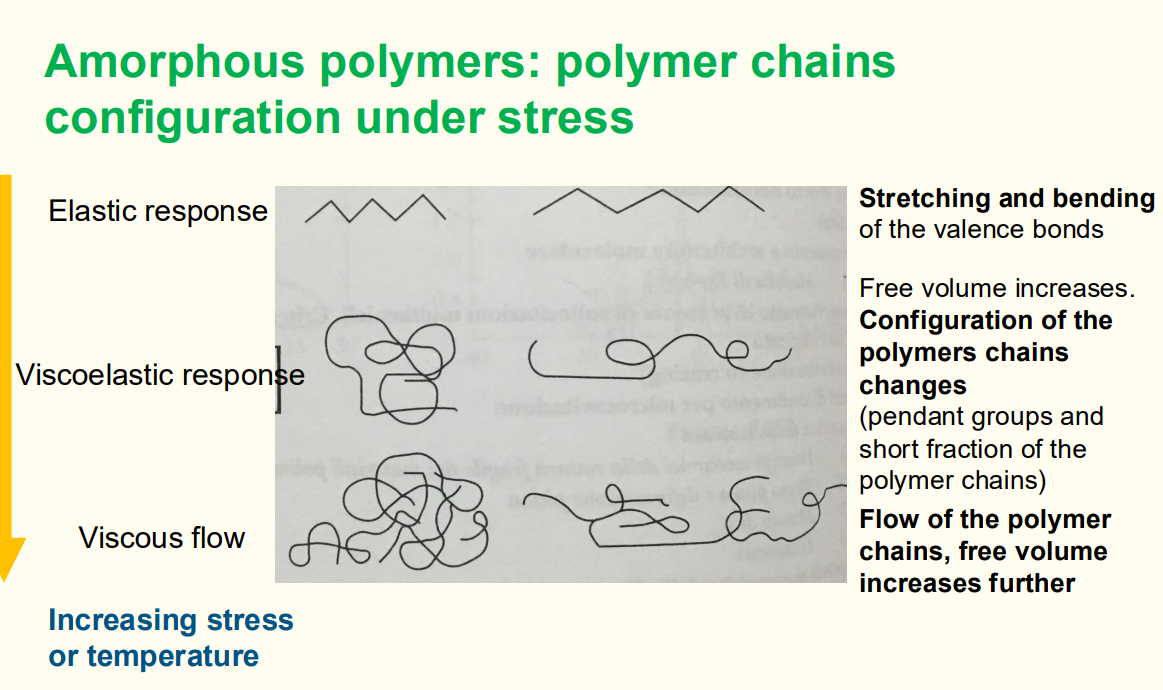
Before stress: Random coil configuration.
After stress:
Elastic response: Chains stretch.
Viscoelastic response: Chains begin to slide past each other.
Viscous flow: Chains flow, leading to permanent deformation