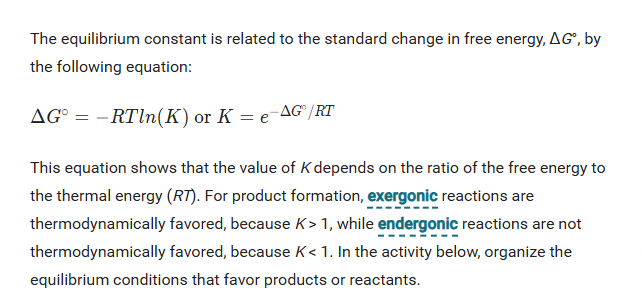
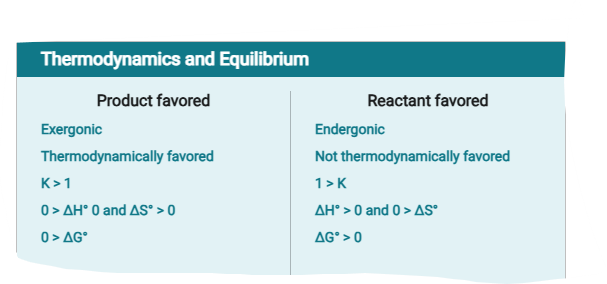
Equilibrium Constant
Temp change, a solid or bubbles forming in a solution indicates that reaction is not at equilibrium.
The equilibrium constant expresses the ratio of products to reactants at equilibrium.
K can be expressed in terms of concentrations or partial pressures. If concentration K(sub)c, partial pressures K(sub)
ICE Tables
Used to compare Q and K(sub)eq, to find if reaction proceeds in forward or reverse direction.
I: Initial concentrations or pressures are determined before the reaction starts
C: Change in concentrations (pressures) for reactants as the reaction moves toward equilibrium.
E: equilibrium concentrations (Pressures) of reactants and products when the system is at equilibrium
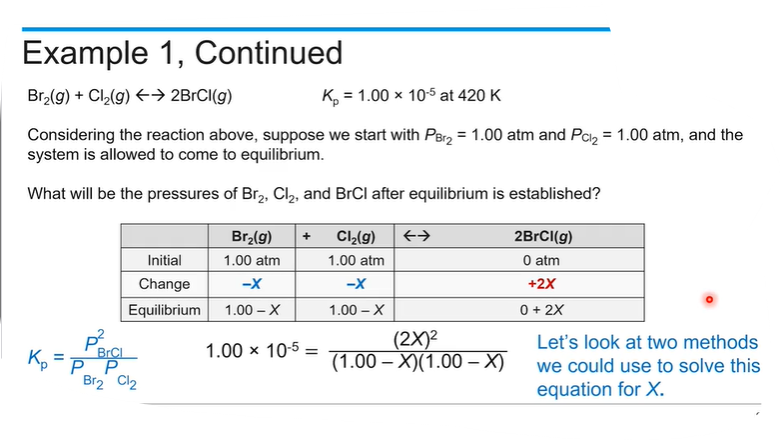
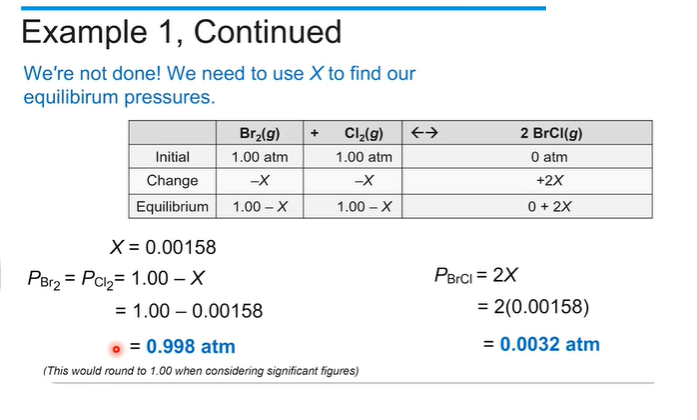
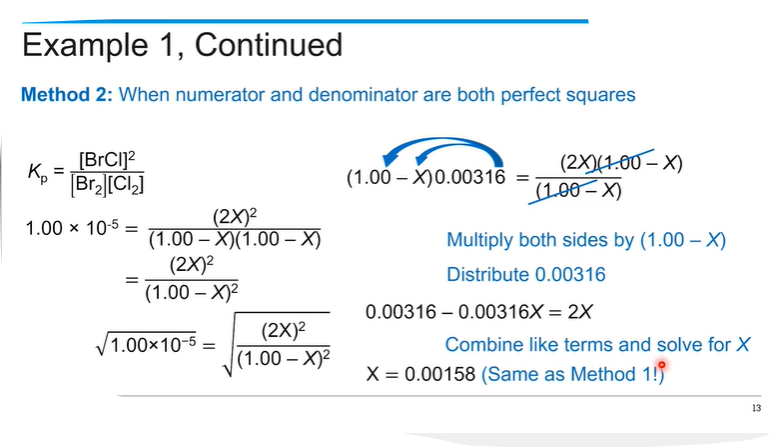
If you know the initial concentration and the equilibrium constant, you can find how much reactant and product there are at equilibrium by making an ICE table.
Start with balanced equation;
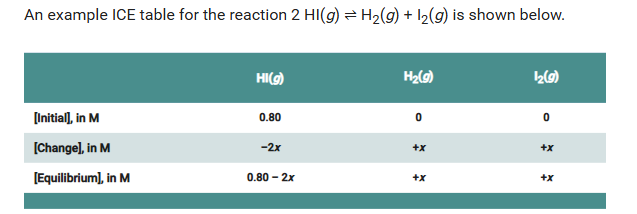
The initial concentration of HI is 0.80 M because 0.40 moles are initially present in the 500.0 mL flask. The initial concentration of each of the products is 0 M. How would you describe the change in concentrations?
Sample Response: 2 HI molecules will be used up for each hydrogen or iodine molecule made. You could write the change as a loss of 2 x from the HI concentration and a gain of x for the hydrogen and iodine concentrations.
Then create the equilibrium expression (K = Products / reactants)based on the stoichiometry of the reaction, which will allow us to relate the concentrations of the reactants and products at equilibrium.
When the equilibrium values from the table are substituted, this equation is

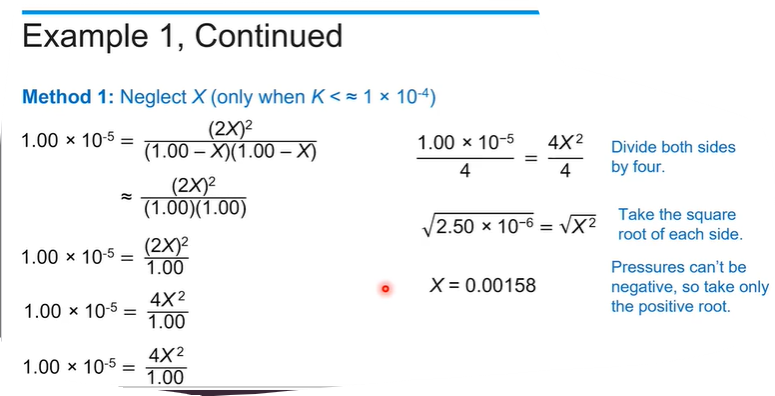
In order to solve the equation for x, what is the next step?
Sample Response: Both the top and bottom of the equilibrium expression are squared, so taking the square root of both sides of the equation is the easiest way to solve for x.

When there is an increase in pressure, the equilibrium will shift towards the side of the reaction with fewer moles of gas. When there is a decrease in pressure, the equilibrium will shift towards the side of the reaction with more moles of gas
a reaction with an equilibrium constant much less than one will not proceed spontaneously in the forward direction; it will favor the reactants, meaning the reaction will tend to proceed spontaneously in the reverse direction instead.
AP Daily
7.3
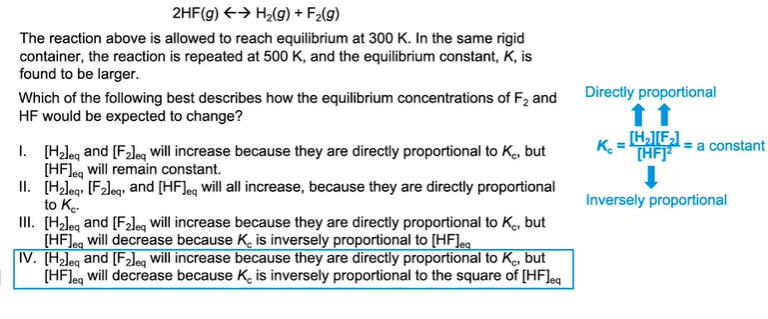
Reaction Quotient, Q
You can represent the relative quantities of reactants and products at any point in time as a ratio of the concentrations of products to concentrations of reactants .
That is the reaction quotient, Q: Products / reactants
Solids and pure liquids are not included in the reaction quotient. Only gases (g) and aqueous solutions (aq) are included.
Q(sub)c [for aqueous] and Q(sub)p [for gases] are not interchangeable, READ CAREFULLY
If there are no products that are applicable to the quotient, then put a 1
Equilibrium Constant, K
When a system is at equilibrium, the constant K is used instead of Q.
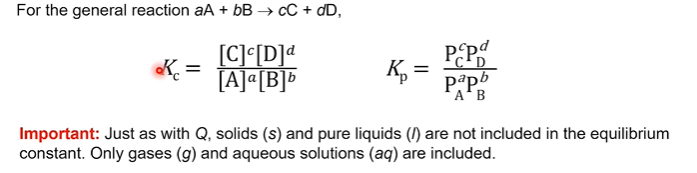
K tells us we are at equilibrium, and Q tells us we are not.
How They Are Related and Significance
The value of Q tells whether the reaction will happen in the forward or reverse direction to reach equilibrium.
The value of K will tell us whether there are more products or reactants at equilibrium.
Temperature and K(sub)EQ / just K
Problems often have a specified temp, due to those values being calculated at specific conditions and temps.
But temp is not needed to calculate K, just concentrations, or pressures.
If temp changes then K will as well.
Extra Info
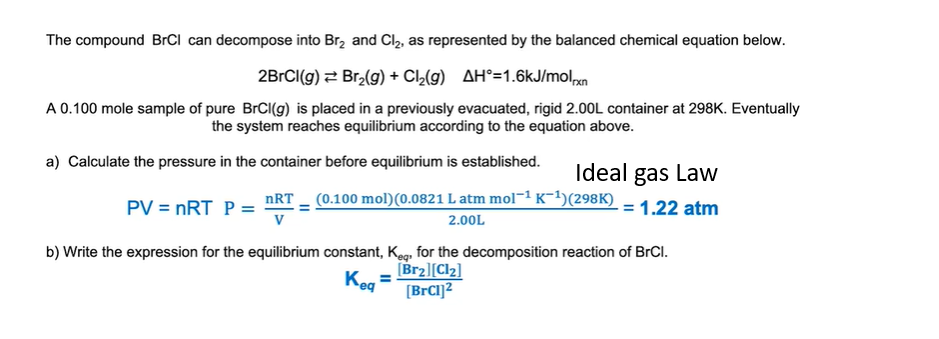
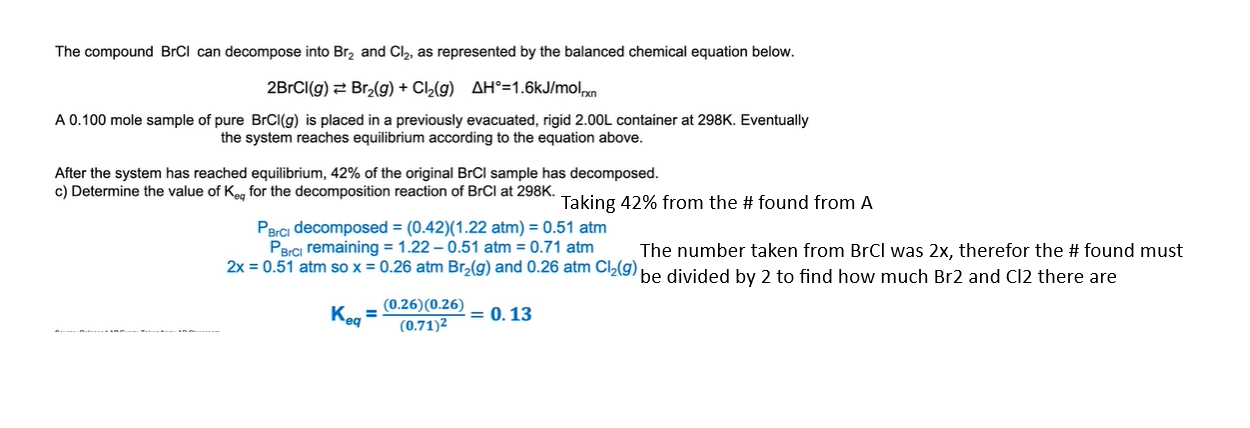
If trying to find the pressure, before equilibrium, use the ideal gas law: PV = nRT and convert it to P = nRT/V.
n = number of moles
R is 0.08206 L atm mol^-1 K-1
T is temp
and V is volume
Magnitude of the Equilibrium Constant
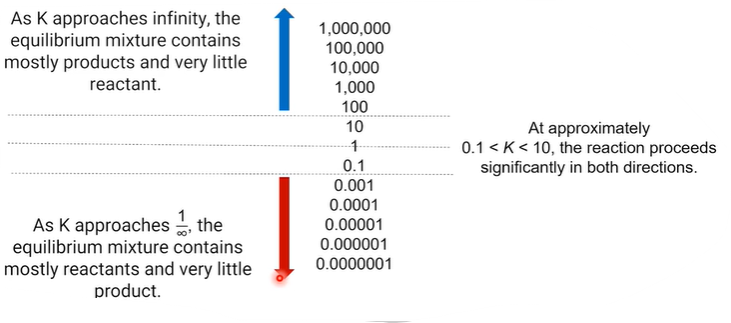
In the equation for the equilibrium constant, the products (numerator) are directly proportional to K, meaning that if products are favored, K will be larger.
Reactants (denominator) are inversely proportional to K, meaning that if reactants are favored, K will be smaller.
If K > 1, there is a higher concentration of products than reactants at equilibrium, so products are favored.
When K (approximately = ) 1 the concentrations of reactants and products are in the same order of magnitude
When K < 1 there are more reactants than products.
7.7: Solving equilibrium problems
If Q is larger than K, the numerator will decrease (and/or denominator increase) until Q =K. Reverse reaction is favored and the concentration of products must decrease, or concentration of reactants increase.
If K > Q numerator must increase. concentration of products must increase, or reactants decrease, forward reaction is favored.
When Q < K, the reaction will proceed in the forward direction