2. biochemistry
polymers vs. macromolecules
- polymers need a chain of molecules with the same base (eg. DNA, amino acids, starch)
- macromolecules are large molecules (eg. carbohydrates, protein, lipids)
- all polymers are macromolecules but not all macromolecules are polymers
The elements of life
- about 20-25% of the 92 elements are required for life
- carbon and hydrogen are required to make organic compounds
- carbon hydrogen oxygen and nitrogen make up 96% of living matter
- nitrogen - ammonia which is found in amino acids
- the remaining 4% consists of calcium, phosphorus, potassium and sulphur
- phosphorus - ATP, and DNA
- trace elements are required in very small amounts
isotopes
- all atoms of an element have the same number of protons but may differ in neutrons. these are isotopes
- radioactive isotopes decay spontaneously, giving off particles of energy
- can be used as diagnostic tools in medicine eg. iodine - thyroid
- radioactive tracing - used to track atoms through metabolism
- radioactive dating - measure dating using time
energy levels of electrons
energy is the capacity to cause change
potential energy is the energy that matter has because of its location or structure
electrons in an atom differ in their amounts of PE
an electron’s state of PE is called its energy level or electron shell
form equals function
- when something is not the right shape, they won’t work
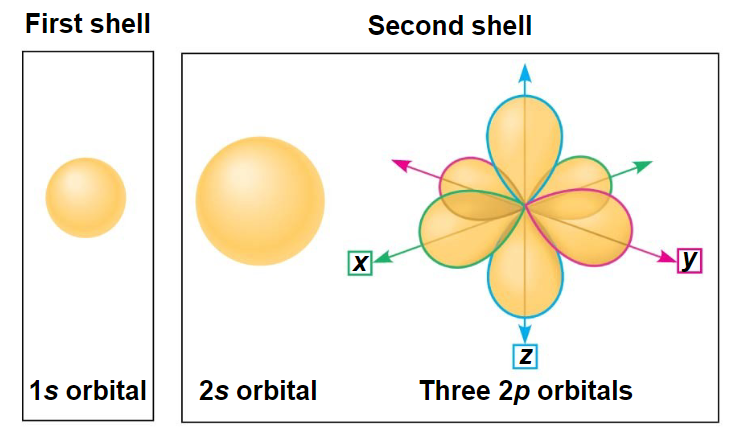
electrons live in orbitals 1s2, 2s2, 2p6
electrons can move between orbitals
- when electrons jump, they emit light
- in photosynthesis, light is used to make electrons jump between orbitals
atoms with incomplete valence shells like to combine
covalent bonds
the sharing of a pair of valence electrons by 2 atoms
a molecule consists of 2 or more atoms held together by covalent bonds
single bonds are the sharing of one pair of electrons
double bonds are the sharing of two pairs of electrons
form equals function
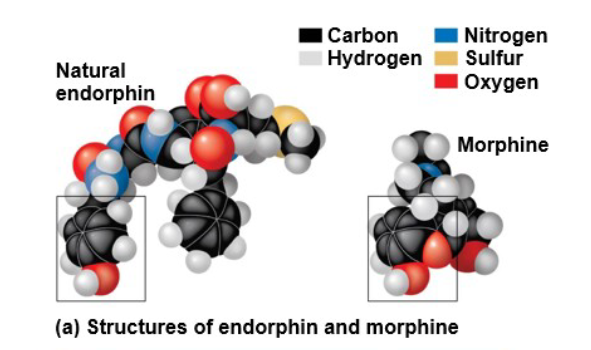
the shape of a molecule determines how it is recognized and responded to
- opiates such as morphine works similarly to endorphins since they have similar shapes and bond to the same receptors in your brain to make you feel good
polarity
water is polar
polar = unsymmetrical, non polar = symmetrical
ionic compounds dissolve in water and only polar molecules
when a compound dissolves in water, the water surrounds the positive atom creating a hydration
cellulose is non-polar despite it being unsymmetrical. this is because when a chain of molecules come together, they become symmetrical which is why water does not dissolve plants
in the covalent bond H2O, oxygen pulls hydrogen’s electron towards it giving oxygen a very small 𝛿- charge and hydrogen a 𝛿+ charge. (delta charge)
H2O has an angular or bent shape because the electrons repel each other
- the angle between the hydrogens is 104.5
methane is non-polar, however, it can dissolve in water under extremely high pressure
electronegativity is an atom’s attraction for the electrons in a covalent bond
- when a covalent bond is formed between 2 identical atoms (H2 or Cl2), the electrons are shared equally since the 2 atoms have the same attraction to the electrons. However, between 2 different elements, one atom will have a larger nucleus and a greater attraction to the electrons.
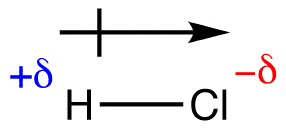 arrow points toward the negatively charged atom, 𝛿+ had lower electronegativity and 𝛿- has greater EN
arrow points toward the negatively charged atom, 𝛿+ had lower electronegativity and 𝛿- has greater ENelectronegativity: 0 - 0.39 = non polar, 0.4 - 1.7 = polar, 1.8 < = ionic
nonpolar bonds share the electrons evenly
in polar bonds, one atom is more electronegative and they do not share the electrons evenly
ionic bonds
- cations are positively charged ions
- anions are negatively charged ions
- ionic compounds are called salts
weak chemical interactions
large biological molecules are held together by weak bonds
intramolecular forces - within a molecule
- covalent, polar covalent and ionic
intermolecular forces - between 2 molecules ↓
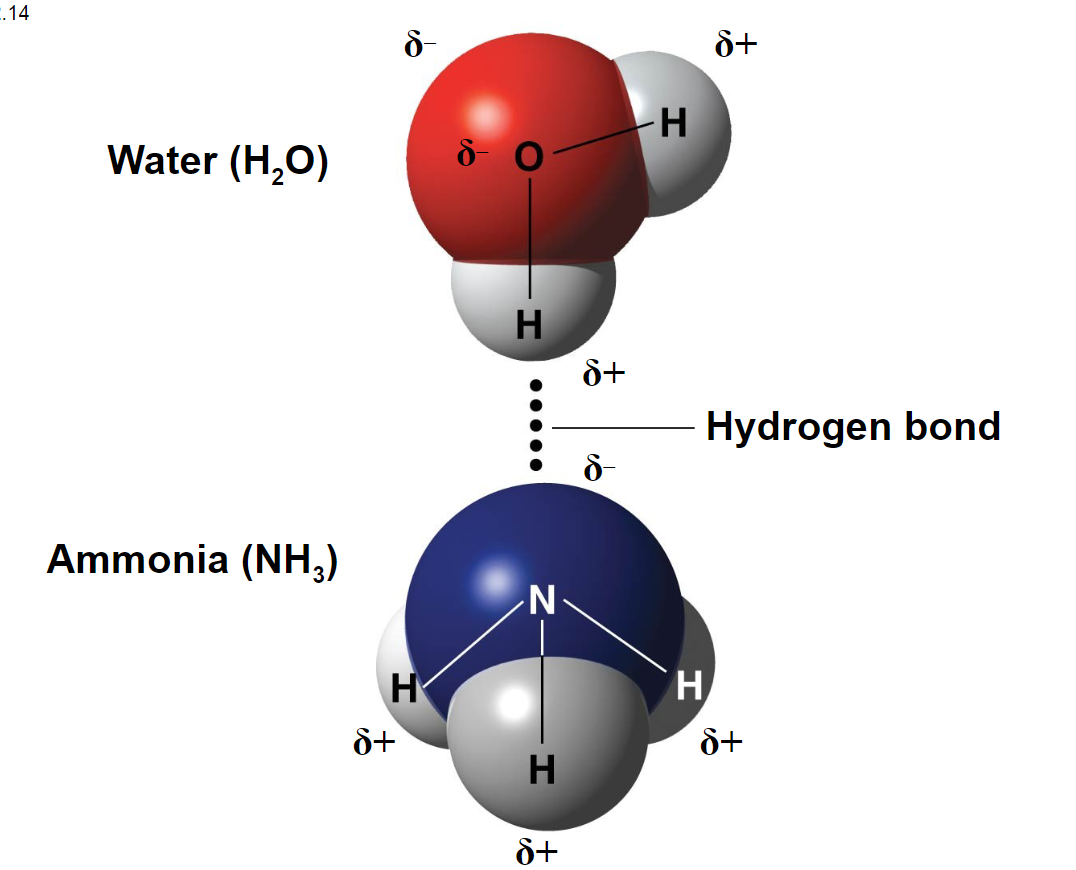
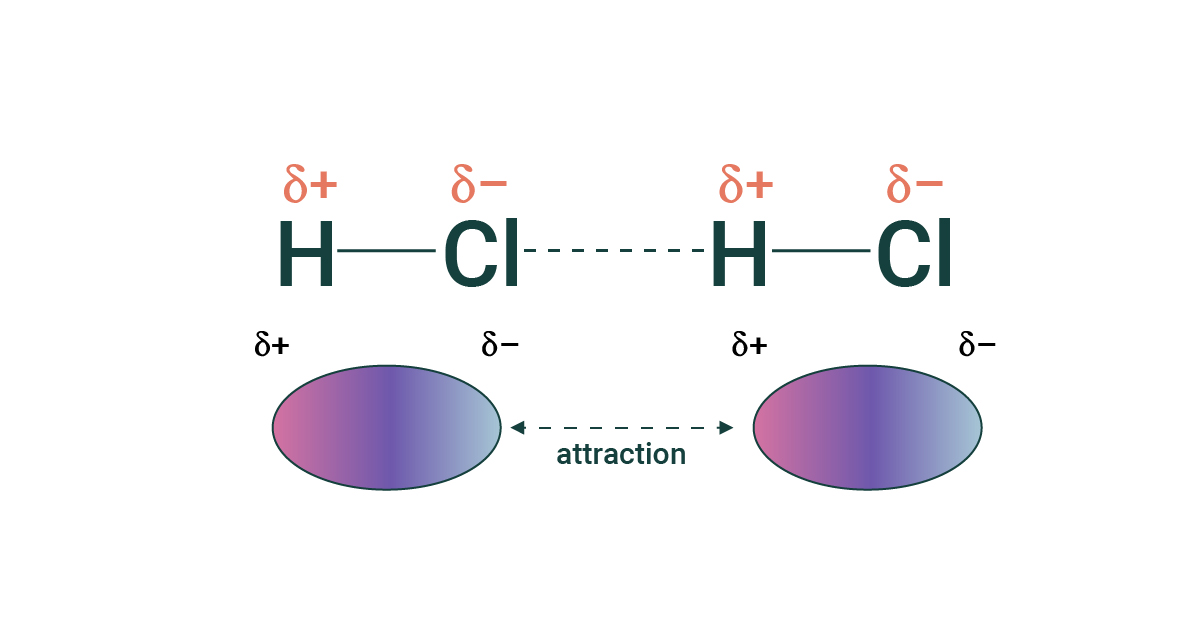
hydrogen bonds - when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom (same as dipole-dipole but much stronger)
- hydrogen bonding is why water is referred to as the universal solvent
- Oxygen, fluorine, nitrogen
Van der Waals interactions - attractions between molecules that are close together as a result of unevenly distributed electrons in a molecule
- electrons are not always evenly distributed and may accumulate in one part of a molecule
- relatively weak attractive forces that act on neutral atoms and molecules and arise because of the electric polarization induced in each of the particles by the presence of other particles.
- london dispersion forces - a temporary force that results when the electrons of 2 atoms occupy positions where there is a temporary dipole force (as the atoms move around). results from a very short separation of charge or dipole that occurs due to temporary electron density fluctuations around atoms or molecules.
- LDF forces are the weakest intermolecular forces and occur between all molecules
- it is the only force acting between non-polar molecules
- the larger the molecules, the stronger the forces
- dipole-dipole forces - when hydrogen bonds with anything other than O, F, N (attractive forces between the positive end of one polar molecule and the negative end of another polar molecule)
- because polar molecules have a net dipole (a positive end and a negative end), the force between oppositely charged ends of polar molecules is a dipole-dipole force
- only exists in polar molecules
chemical reactions
- the making and breaking of chemical bonds
- eg. photosynthesis: 6 CO2 + 6 H2O → C6H12O6 + 6 O2
- all chemical reactions are reversible
- chemical equilibrium is reached when the forward and reverse reactions occur at the same rate