Lecture 9
Metabolism
Nutritional Components of Microbes
Substrates and Nutrients: Essential inorganic and organic elements required by microbes, primarily carbon (C), nitrogen (N), oxygen (O), and sulfur (S), alongside various vitamins that serve as co-factors in metabolic reactions.
Precursors: A set of twelve major building blocks, including amino acids, fatty acids, and nucleotides, crucial for synthesizing macromolecules like proteins and nucleic acids.
Macromolecules: Include critical biological structures such as proteins (for catalysis and structure), polysaccharides (for energy storage and structural integrity), nucleic acids (for genetic information), and lipids (for membrane formation).
Supramolecular Structures: Complex assemblies such as enzymatic systems, ribosomes, and the nucleoid which facilitate cellular function and organization.
Level of organization (order): Bottom to top: substrates/nutrients, precursors, monomers, macromolecules, supramolecular structures, cell.
Essential Metabolic Demands
All organisms necessitate:
Source of Energy, electrons, and carbon:
Phototrophs: Light, H2O, H2S, and CO2
GIT Bacteria: Carbohydrates, Carbohydrates, carbohydrates. Most bacteria in GI ferment carbs as primary energy source for microbial growth.
Definitions of Energy
Energy: The capacity for doing work, signifying the transfer of energy within a system that induces physical or chemical changes.
Forms of Energy: Include thermal, mechanical, electrical, osmotic, chemical, and gravitational energy.
Kinetic Energy: The energy of an object in motion.
Potential Energy: The stored energy within a system, which can be released to do work later.
Equations: E=mc²: energy, mass, and the speed of light, illustrating energy-mass equivalence in physical systems.
Energy Conservation in Microbial Cells
Reaction Equations: Explains how cells harness energy through catabolic and anabolic pathways.
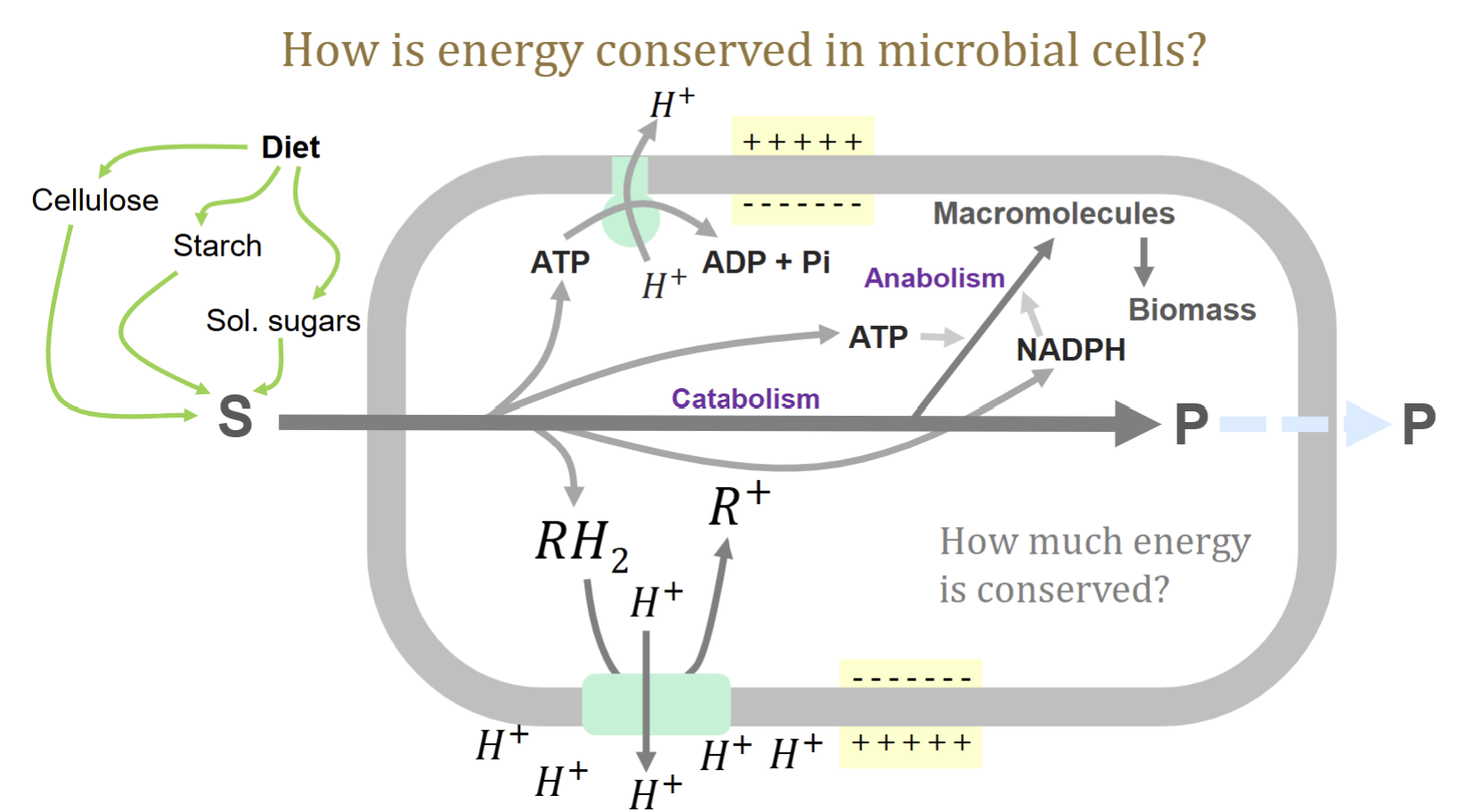
Role of ATP, NADPH: Both are pivotal in anaerobic cellular processes, functioning as energy carriers and reducing agents in biochemical reactions.
Bioenergetics
Definition: The biology of energy transformations and energy exchanges within and between living things and their
environments.
Importance of Photosynthesis: Key to generating potential energy (CHOs) that can be used by animals and microbes.
Laws of Thermodynamics - Energy Conservation
First Law: Energy cannot be created or destroyed; it can only be transformed from one form to another
Laws of Thermodynamics - Directionality
Second Law: States that in spontaneous processes, entropy increases, demonstrating the natural tendency toward disorder and the inherent inefficiencies in energy transduction within organisms.
Free Energy & Chemical Potential
Many energy transactions in a cell are chemical in nature
Free Energy (G): A crucial thermodynamic quantity that indicates a system’s ability to perform work at constant temperature and pressure.
Measured experimentally by calorimetric methods
Change indicates spontaneity of a process
Calculation: ΔG = ΔH - TΔS, where ΔH represents the change in enthalpy (total energy), T is the temperature, and ΔS is the change in entropy
Deriving Free Energy from Metabolic Reactions
A + B → C + D → ΔG0 = - RT ln Keq
ΔG = Keq under standard conditions: temp 298 K, 1M concentrations, H+ is pH 7, and R is constant at 8.314.
Shows how free energy changes are determined based on concentrations of reactants/products and temperature, important for metabolic regulation.
Equilibrium Constants and Free Energy Changes
Relationship Between K’eq and ΔG°’ Values: A comprehensive table exhibiting how equilibrium constants relate to free energy changes under standard conditions, emphasizing the conditions under which reactions can proceed in a forward or reverse manner. As products increase in a reaction, reagents decrease.
Metabolism Fundamentals
Metabolism:
Catabolism: Characterized by exergonic reactions (ΔG’0 < 0) that release energy, making it available for biological work.
Anabolism: Encompasses endergonic reactions (ΔG’0 > 0) that consume energy for biosynthesis.
Emphasis on how coupled reactions (ΔG’0<0) can drive non-spontaneous processes
Role of ATP in Metabolism
ATP Significance: Acts as a primary energy intermediary in cells, crucial for coupling metabolic reactions, with examples illustrating how ATP facilitates energy transfer in various biochemical pathways.
Hexokinase Reaction Example
Hexokinase Reaction:
Reaction: Glucose + Pi → Glucose-6P + H2O.
The equilibrium constant indicates whether the reaction is spontaneous through ΔG° calculations, highlighting the role of this enzyme in metabolic pathways.
Coupled Reactions
Mechanism of Coupling: Explores how spontaneous reactions can facilitate the occurrence of non-spontaneous ones through additive free energy changes.
Glucose + ATP → Glucose-6P + ADP
Free energy changes of couple reactions are additive
Directionality in Reactions
Importance of ATP hydrolysis and glucose phosphate formation in driving various metabolic pathways, Pi is highly stable and should not be released into solution:
Low energy: Glu + ATP → ADP + Glu-6P (-kj/mol opposite direction of reaction)
Medium energy: Glu + Pi → Glu-6P +H2O
High energy: ATP + H2O → ADP + Pi
Free Energy from Redox Potentials
Equations Relating Redox Potentials: Thermodynamics of redox reactions, shows how specific electron acceptors/donors provide energetic advantages influencing microbial metabolism.
Δ𝑮′𝒐= −nFΔE’0
Microorganisms capable oof using acceptors with a more positive electrode potential have a thermodynamic advantage (greater capacity to do work).
Bioenergetics in Microbial Communities
Overview of Role Differentiation: Elucidates the roles of Primary Degrader, Primary Fermenter, and Secondary Fermenter in microbial communities, supporting functional diversity.
 Knowt
Knowt