Atomic structure
Definitions:
==Atomic structure== = number of protons in the nucleus of an atom
==Mass number== = number of protons and neutrons in the nucleus of an atom
Nuclear charge = total charge of all protons in the nucleus
Isotopes = atoms with the same number of protons and a different number of neutrons
RAM = average mass of an atom of an element, relative to 1/12th of the mass of a carbon-12 atom
RIM = average mass of a isotope relative to 1/12th of the mass of an atom of carbon-12
RMM = average mass of a molecule relative to 1/12th of a carbon-12 atom
Quantum shells = the energy levels of an electron
First ionisation energy = the energy required to remove 1 mole of electrons from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions
Periodicity = trends in element properties with increasing atomic number
Hunds rule = when electrons fill orbitals, the fill up singly before they pair up
Pauli exclusion principle = electrons in the same orbital must be in opposite spin
Aufbaus principle = as the atomic number increases, electrons are added from the lowest energy level (except from Cr, Cu)
The atom:
| Particle | mass relative to a proton | charge relative to a proton | Position of atom |
|---|---|---|---|
| Proton | 1 | +1 | nucleus |
| neutron | 1 | 0 | nucleus |
| electron | 1/1840 | -1 | shells |
Mass spectrometry
- mass spectrometry separates atoms and molecules according to their masses
- Atoms are bombarded with electrons from a charged gun and they’re ionised to form 1+ ions = ==ionisation==
- Ions are charged and therefore can be accelerated by a electric field = ==acceleration==
- Charged particles are deflected by a magnetic field = ==deflection==
- Particles are detected by photographic methods = ==detection==
the output from a detector is presented as a stick diagram and shows the mass to charge ration of the ions and the relative abundance
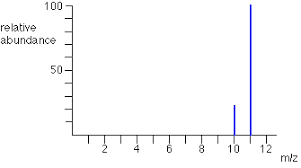
These show isotopic masses as they give the relative mass of particular isotopes
Calculations of isotopic masses:
E.g.
Mg 24 = 78.6%
Mg 25 = 10.1%
Mg 26 = 11.3%
- (24 x 78.6) + (25 x 10.1) + (26 x 11.3) = 2432.7
- 2432.7/100 = 24.3
- ==remember with molecules, e.g. O2, to multiply by 2==
Electrons and energy levels:
The main sub shells of atoms are S, P, D, and F
2 electrons can be held in the S orbital
6 electrons can be held in the P orbital
10 electrons can be held in the D orbital
14 electrons can be held in the F orbital
orbitals are areas where one is likely to find electrons at any point
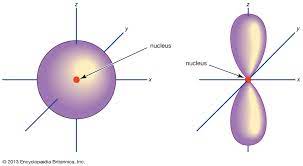
The energy levels are organised as follows:
%%1s2s2p3s3p4s3d4p4d4f…….%%
The 3d orbital is after the 4s orbital when filling up the energy levels because it has a lower energy level, however, when losing electrons, the electrons are lost from 4s before 3d
there are 3 rules in filling up energy levels
- Electrons go into the orbital with the lowest available energy level
- Each sub-orbital can only contain 2 electrons of opposite spin
- When there are 2 or more orbitals in the same energy level, the fill up individually before being paired
Electron structure and the periodic table:
- the periodic table is arranged in order of atomic number
- Horizontal rows = periods
- Vertical rows = groups
- The S block = group 1 & 2 (last electrons are in the S orbital)
- The P block = group 3,4,5,6,7&0 (last electrons are in the P orbital)
- The D block = period 4,5,6&7 between group 2 & 3 (last electrons are in the d orbital)
Periodic trends:
Melting points of the elements
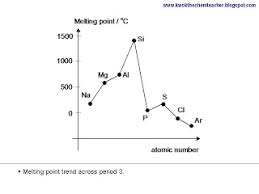
- the melting point increases from Na to Si due to an increase strength in the metallic bonding due to the increase of charge and therefore stronger nuclear attraction
- The melting point decreases from Si to Ar due to Si having the highest melting point because of its giant molecular structure
- The strength of London dispersive forces is dependant on the number of electrons, and as argon is a single atom held together by weak London forces, it has the lowest melting point
Ionisation energies:
Definition = the energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous 1+ ions
- X(g) → X+(g) + e-
The second ionisation energy would look like this:
- X+(g) → X2+(g) + e-
- Successive IE of an element gets larger every time as an electron is being removed and therefore the charge is increasing = stronger attraction and therefore it is harder to remove another electron from the formed ion

- There is a general increase across a period before the value drops dramatically at the start of another period
- The values get smaller down groups as the electrons removed comes from an orbital further from the nucleus and therefore there is more shielding
- There is a drop in the value for boron. This is because the extra electrons has gone into one of the 2p orbitals, hence increasing the shielding, making the electrons easier to remove
- There is a drop in the value for oxygen. As the extra electron has paired up with one the the electrons already in one of the 2p orbitals and therefore the repulsive forces between the two electrons means that less energy is required to remove one of them
Factors that affect IE:
- ==the size of the nuclear charge== - as the charge increases, the attraction for the outer electrons increases and therefore increases IE
- ==The distance from the outer electron to the nucleus== - as distance increases and therefore the attraction from the positive nucleus to the electrons decreases and therefore this decreases IE
- ==The shielding effect of electrons== - electrons in inner shells have a repelling effect on the outer shells of an atom. This reduces the attraction between the nucleus and the electrons in the outer shell, therefore this decreases the IE