Chapter 2: The Chemical Level of Organization
Chemicals called nucleotide bases are the foundation of the genetic code with the instructions on how to build and maintain the human body from conception through old age.
About three billion of these base pairs in human DNA.
ELEMENTS AND ATOMS: THE BUILDING BLOCKS OF MATTER (CHAPTER 2.1)
The substance of the universe - from a grain of sand to a star is called matter.
Scientists define matter as anything that occupies space and has mass.
An object’s mass is the amount of matter contained in the object.
The object’s mass is the same whether that object is on Earth or in the zero-gravity environment of outer space.
An object’s weight, on the other hand, is it mass as affected by the pull of gravity.
Element
A pure substance that is distinguished from all other matter by the fact that it cannot be created or broken down by ordinary chemical means.
Calcium is essential to the human body; it is absorbed and used for a number of processes, including strengthening bones.
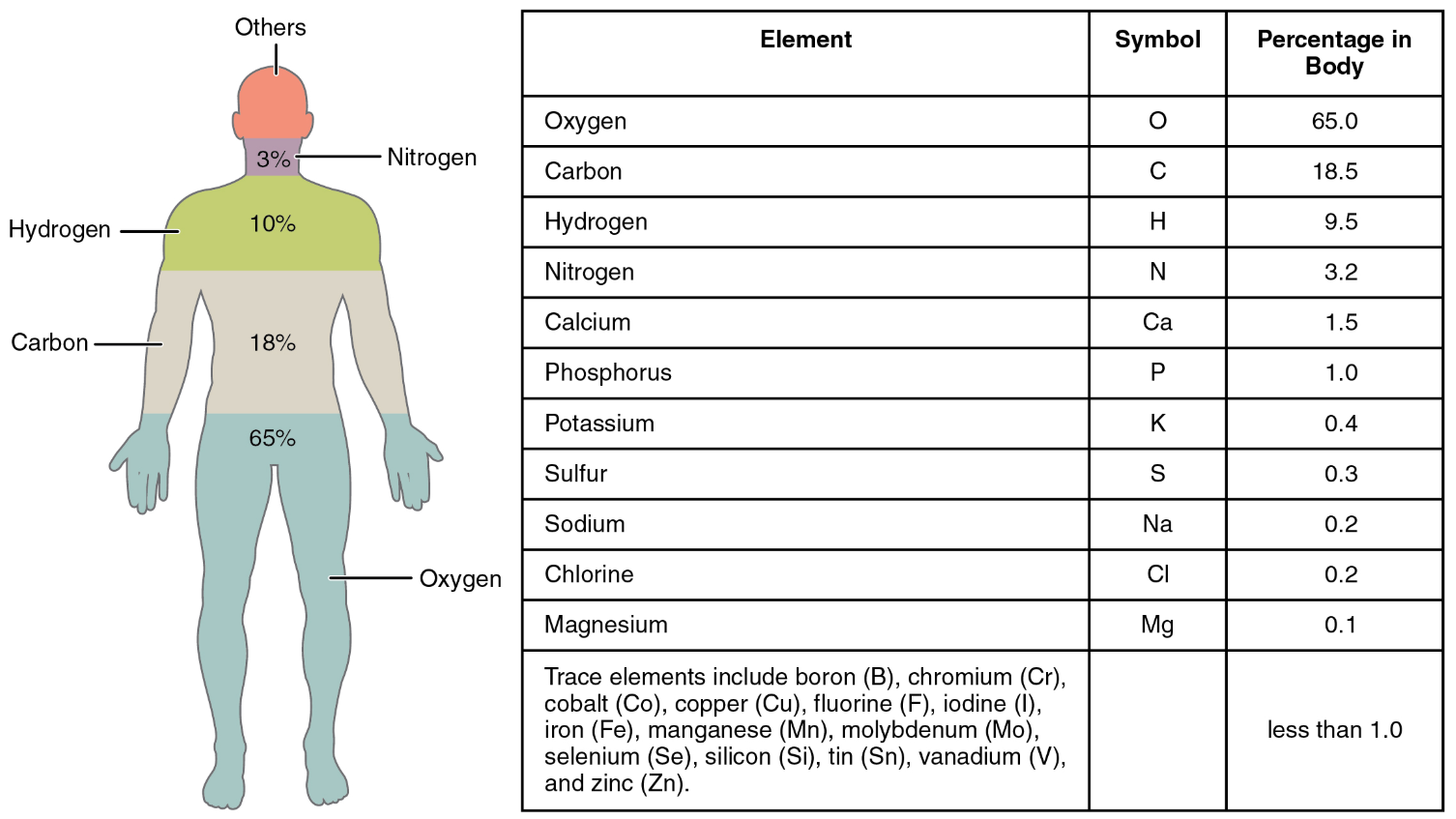
Compound
Is a substance composed of two or more elements joined by chemical bonds.
Atom
The smallest quantity of an element that retains the unique properties of that element.
Atoms are made up of even smaller subatomic particles, three types of which are important: proton, neutron, and electron.
The number of positively-charged protons and non-charged (“neutral) neutrons, gives mass to the atom, and the number of protons determines the elements.
The number of negatively-charged electrons that “spin” around the nucleus at close to the speed of light equals the number of protons.
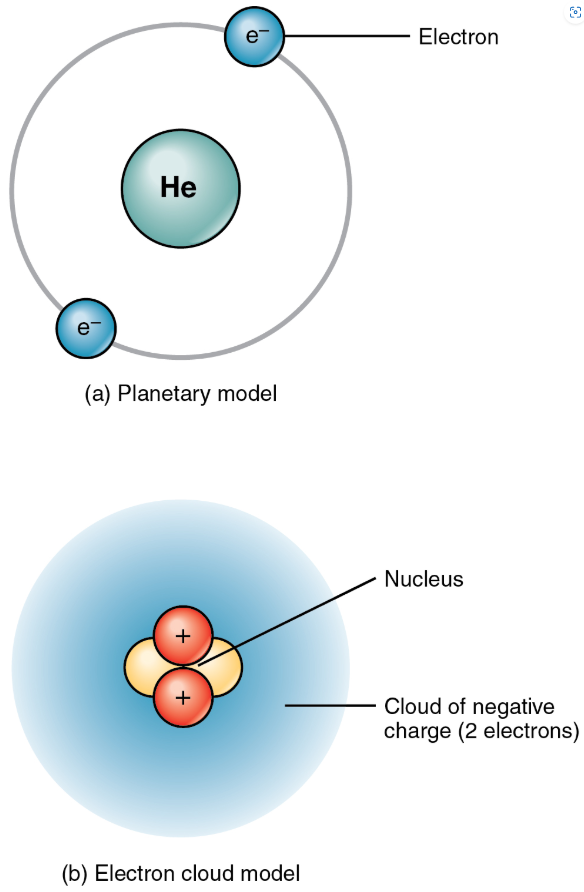
An atom’s protons and electrons carry electrical charges.
Protons with their positive charge, are designated p+.
Electrons which have a negative charge, are designated e-.
An atom’s neutrons have no charge: they are electrically neutral.
The positively charged protons attract the negatively charged electrons.
Gives the atom some structural stability.
The number of protons and electrons within a neutral atom are equal, thus, the atom’s overall charge is balanced.
Atomic number
Is the number of protons in the nucleus of the atom, identifies the element.
Am atom usually has the same number of electrons as protons, the atomic number identifies the usual number of electrons as well.
In their most common form, many elements also contain the same number of neutrons as protons.
An element’s mass number is the sum of the number of protons and neutrons in its nucleus.
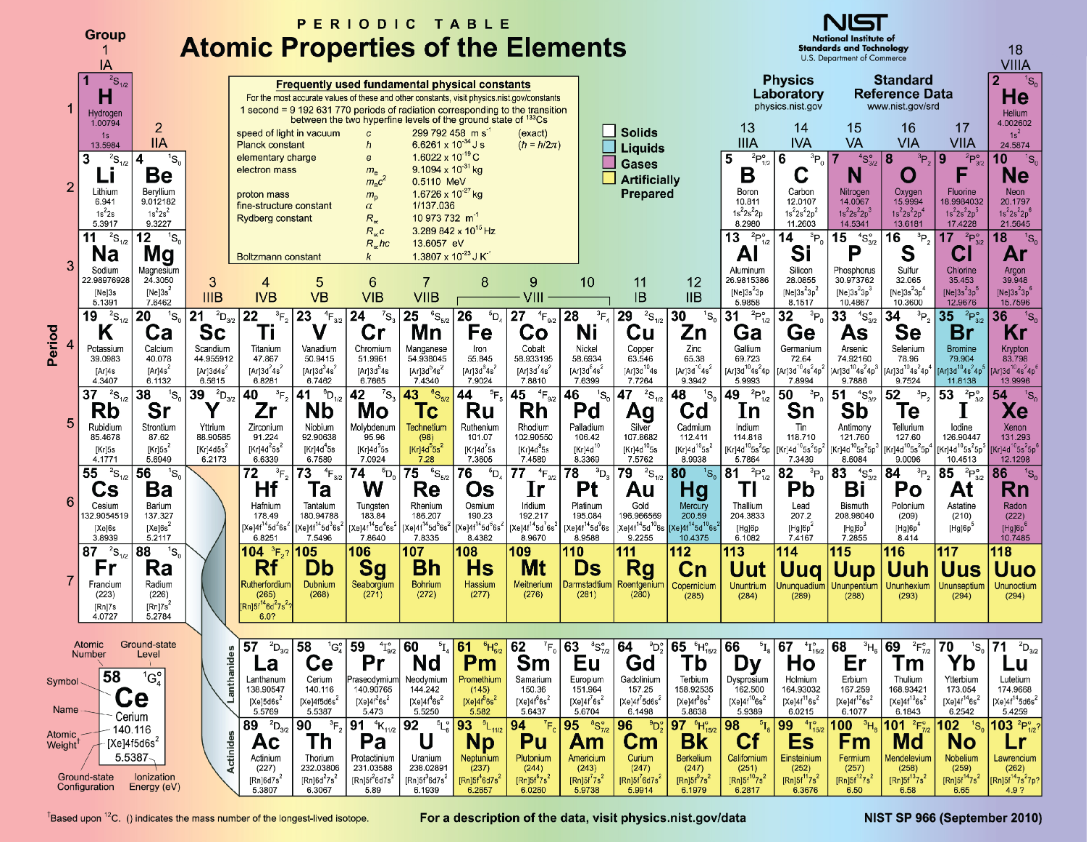
Periodic table of the elements
Is a chart identifying the 92 elements found in nature, as well as several larger, unstable elements discovered experimentally.
The elements are arranged in order of their atomic number.
Isotope
One of the different forms of an element, distinguished from one another by different numbers of neutrons.
Hydrogen has three common isotopes
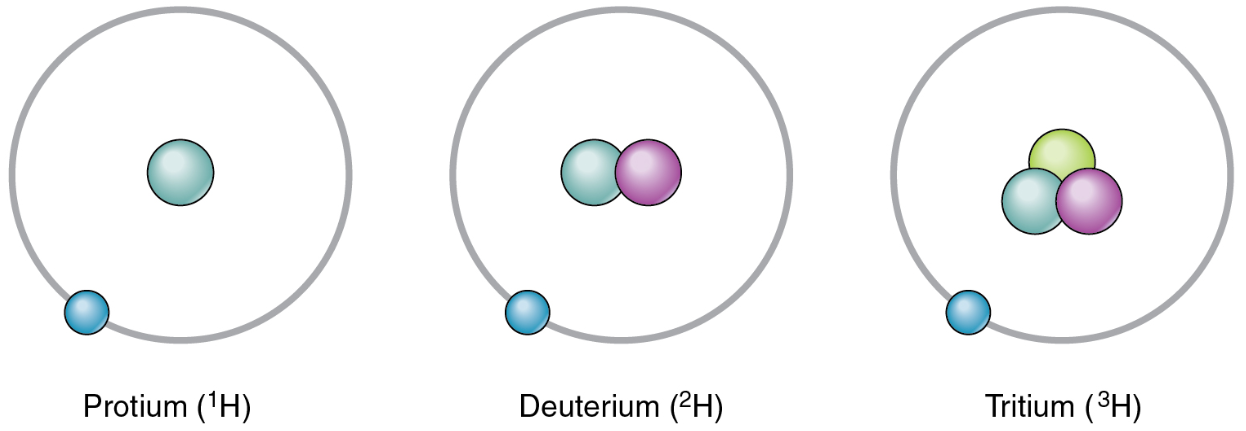
An isotope that contains more than the usual number of neutrons is referred to as a heavy isotope.
Have isotopes tend to be unstable, and unstable isotopes are radioactive.
Radioactive isotope
An isotope whose nucleus readily decays, giving off subatomic particles and electromagnetic energy.
Different radioactive isotopes (also called radioisotopes) differ in their half-life, the time it take for half of any size sample of an isotope to decay.
Electron shell
A layer of electrons that encircle the nucleus at a distinct energy level.
The atoms of the elements found in the human body have from one to five electrons shells, and all electron shells hold eight electrons except the first shell, which can only hold two
This configuration of electron shells is the same for all atoms.
The factor that most strongly governs the tendency of an atom to participate in chemical reactions is the number of electrons in its valence shell.
Valence shell
An atom’s outermost electron shell.
If the valence shell is full, the atom is stable; meaning its electrons are unlikely to be pulled away from the nucleus by the electrical charge of other atoms.
If the valence shell is not full, the atom is reactive; meaning it will tend to react with other atoms in ways that make the valence shell full.
Most atoms are most stable when there are exactly eight electrons in their valence shell.
This principle is referred to as the octet rule, and it states that an atom will give up, gain, or share electrons with another atom so that it ends up with eight electrons in its own valence shell.
CHEMICAL BONDS (CHAPTER 2.2)
Atoms separated by a great distance cannot link; rather, they must come close enough for the electrons in their valence shells to interact.
Atoms link by forming a chemical bond.
Bond
A weak or strong electrical attraction that holds atoms in the same vicinity.
The new grouping is typically more stable — less likely to react again — than its component atoms were when they were separate.
Molecule
A more or less stable grouping of two or more atoms held together by chemical bonds.
Chemical compound
When a molecule is made up of two or more atoms of different elements.
Three types of chemical bonds are important in human physiology, because they hold together substances that are used by the body for critical aspects of homeostasis, signaling, and energy production, to name just a few important processes.
These are:
Ionic bonds
Covalent bonds
Hydrogen bonds
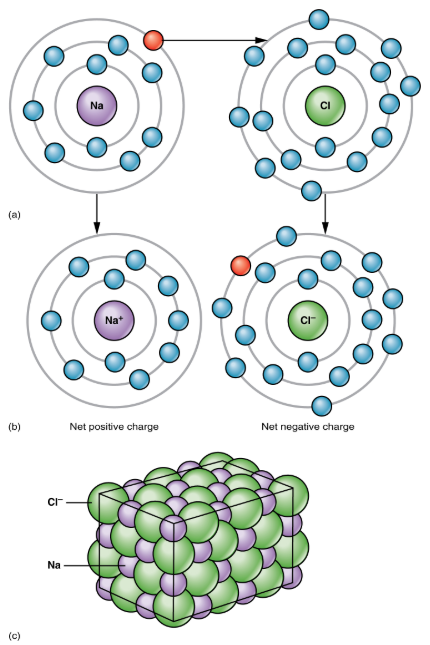
Atom typically has the same number of positively charged protons and negatively charged electrons.
As long as this situation remains, the atom is electrically neutral.
When an atom participates in a chemical reaction that results in the donation or acceptance of one or more electrons, the atom will then become positively or negatively charged.
This happens frequently for most atoms in order to have a full valence shell.
This can happen either by gaining electrons to fill a shell that is more than half-full, or by giving away electrons to empty a shell that is less than half-full, thereby leaving the next smaller electron shell as the new, full, valence shell.
Ion
An atom that has an electrical charge — whether positive or negative.
Cation
A positively charged ion
Anion
A negatively charged ion
Atoms that have more than one electron to donate or accept will end up with stronger positive or negative changes.
The opposite charges of cations and anions exert a moderately strong mutual attraction that keeps the atoms in close proximity forming an ionic bond.
Ionic bond
An ongoing, close association between ions of opposite charge.
Water is an essential component of life because it is able to break the tonic bonds in salts to free the ions.
Electrolytes
The electrical activity that derives from the interactions of the charged ions
Covalent bonds
Unlike ionic bonds formed by the attraction between a cation’s positive charge and an anion’s negative charge, molecules fromed by a covalent bond share electrons in a mutually stabilizing relationship.
Electrons move back and forth between the elements.
Two covalently bonded atoms typically share just one or two electron pairs, though larger sharings are possible.
In covalent bonds, electrons in the two atoms’ overlapping atomic orbitals are shared to fill the valence shells of both atoms, ultimately stabilizing both of the atoms involved.
A Single covalent bonds, a single electron pair is shared between two atoms, while in a double covalent bond, two pairs of electrons are shared between two atoms.
There even are triple covalent bonds, where three electron pairs are shared between two atoms.
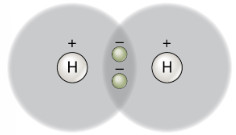
(a) A single covalent bond: hydrogen gas (H—H). Two atoms of hydrogen each share theiri solitary electron in a single covalent bond.
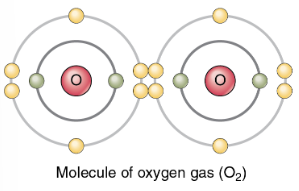
(b) A double covalent bond: oxygen gas (O=O). An atom of oxygen has six electrons in its valence shell; thus, two more would make it stable. Two atoms of oxygen achieve stability by sharing two pairs of electrons in a double covalent bond.
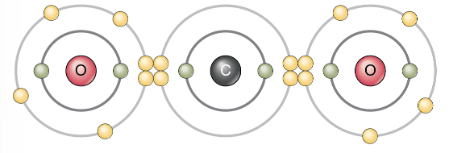
(c) Two double covalent bonds: carbon dioxide (O=C=O). An atom of carbon has four electrons in its valence shell; thus four more would make it stable. An atom of carbon and two atoms of oxygen achieve stability by sharing two electron pair each. In two double covalent bonds.
The sharing of the negative electrons is relatively equal, as is the electrical pull of the positive protons in the nucleus of the atoms involved.
Groups of legislator with completely opposite views on a particular issue are often described as “polarized”
Polar molecule
A molecule that contains regions that have opposite electrical changes.
Polar molecules occur when atoms share electrons unequally, in polar covalent bonds.
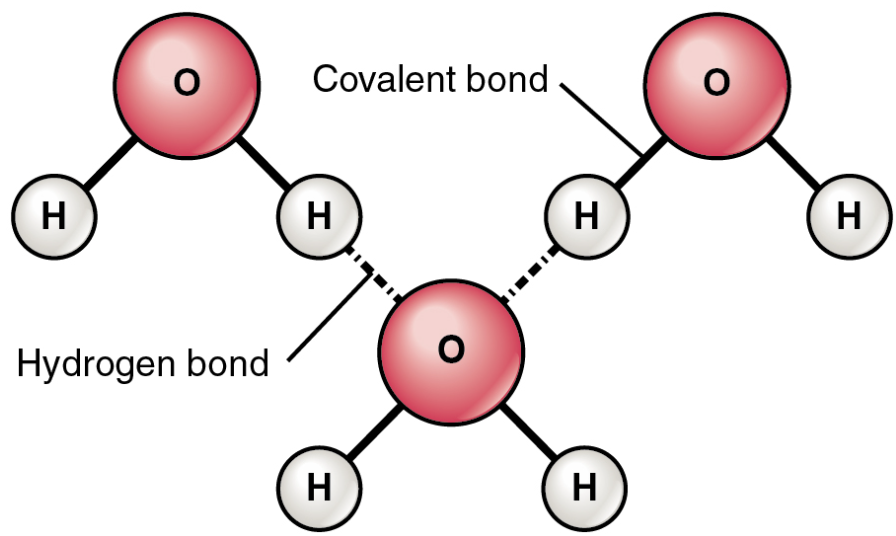
Hydrogen Bond
Formed when a weakly positive hydrogen atom already bonded to one electronegative atom is attracted to another electronegative atom from another molecule.
CHEMICAL REACTIONS (CHAPTER 2.3)
One characteristic of a living organism is metabolism
Whiich is the sum total of all of the chemical reactions that go on to maintain that organism’s helath and life.
Anabolic chemical reactions;
They form larger molecules from smaller molecules or atoms.
Metabolism can proceed in another direction: Catabolic chemical reactions,
Bonds between components of larger molecules break, releasing smaller molecules or atoms.
Both types of reaction involve exchanges not only matter, but of energy.
Kinetic energy
The form of energy powering any type of matter in motion. Imagine you are building a brick wall.
Potential energy
The energy of position, or the energy matter possesses because of the positioning or structure of its components.
Potential energy is stored in the bonds between atoms and molecules.
Chemical energy
Form of potential energy in which energy is stored in chemical bonds.
All energy, is neither created nor destroyed; rather, it is converted from one form to another.