Chapter 8 - Covalent Bonding
8.1 - Molecular Compounds
bond - electrons are shared/transferred in the valence shell
ionic bond - an electron from 1 valence shell is transferred to another
covalent bond - share electrons in valence shell
molecule - neutral (p = e) group of atoms that are joined by covalent bonds
diatomic molecule - same atoms of an element that are covalently bonded
molecular compound
diff atoms that are covalently bonded
low melting/boiling points compared to ionic compounds which have high melting/boiling points
molecular formula - tells you…
number of atoms in a molecule
number of molecules
types of atoms
H2O
1 water molecule
2 hydrogen
1 oxygen
2:1
C6H12O6
1 glucose molecule
6 carbon
12 hydrogen
6 oxygen
1:2:1
chg in composition or ratio → chg in substance
a straight line represents one bond/one pair of electrons
TYPES OF FORMULAS

electron sharing usually occurs so atoms can attain the electron configuration of noble gases
groups 4 - 7a are more likely to form covalent bonds
single covalent bond - 1 shared pair of electrons
double - 2
triple - 3
8.2
coordinate covalent bond - 1 atom contributes both bonding electrons
(represented by →)
polyatomic ion - tightly bound group of atoms that have a charge
bond dissociation energy - energy needed to break one mole of bonds (2 molecules covalently bonded)
resonance structure - occurs when it’s possible to write 2+ valid electron dot structure with the same number of electron pairs for a molecule/ion (way to envision double bonding)
(represented by double headed arrows)
main exceptions to octet rule
ClO2
NO
8.3 - Bonding Theories
molecular orbital - atomic orbital overlap during chemical bonds
bonding orbital - a molecular orbital in covalent bonds that can have 2 electrons occupy it
just as atomic orbitals belong to particular atoms, molecular orbitals belong to molecules as wholes
sigma bond - 2 atomic orbitals that combine to form a molecular orbital that is symmetrical around an axis connecting the 2 atomic nuclei

pi bond
bonding electrons not as likely to be found in the sausage shaped regions above and below the bond axis
not symmetrical
don’t overlap as much as sigma
weaker than sigma

VSEPR Theory
valence shell electron pair repulsion theory
explains 3D shape
repulsion bc electron pairs cause molecular shapes to adjust so that the valence electron pairs satay as far apart as possible
tetrahedral angle - 109.5°
hybridization - several atomic orbitals mix and form same total number of hybrid orbitals
orbital hybridization provides info about both molecular bonding and shape
single bond stronger than double
double stronger than triple
ane - single bond between C
ene - double bond between C
yne - triple bond between C

8.4 - Polar Bonds and Molecules
nonpolar covalent bond
bonding electrons shared equally
EX: H2, O2, N2, CO2
polar covalent bond - electrons shared unequally
polar molecule - 1 end is slightly negative, other is slightly positive
EX: H2O
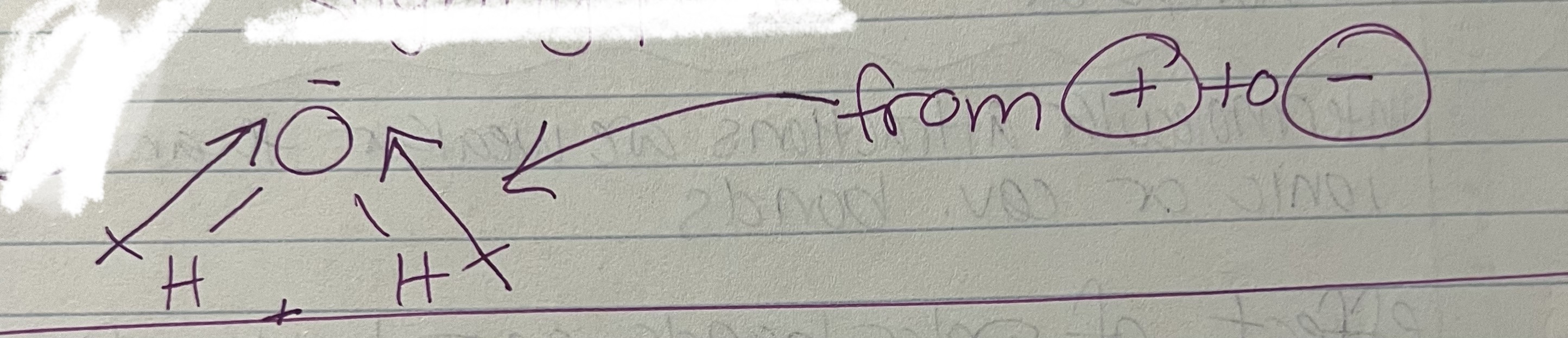
high EN - negative
low EN - positive
electronegativity - attractive force of an atom for electrons
if (EN diff. rang) then it is (bond):
0-0.4; nonpolar covalent
0.4-1; moderately polar covalent
1-2; very polar covalent
2+; ionic
dipolar molecule (dipole) - molecule that has 2 poles
intermolecular attractions are weaker than either ionic or covalent bonds
effect of polar bonds on polarity of an entire molecule → depends on shape of molecule and orientation of polar bonds

van der Waals forces
weakest attraction of molecule to molecule
weaker than ionic and covalent bonds
consists of dipole interactions and dispersion forces
dipole interactions
polar molecules are attracted to one another
weaker than ionic bonds
dispersion forces
weakest of all molecular interactions
caused by motion of electrons
occurs even between non polar molecules
high strength → high number of electrons in molecules
halogen diatomics
Cl2
F2
Br2
I2
attracts mainly bc of dispersion forces
H Bonds
attractive forces that covalently bond to a very electronegative atom
also weakly bonded to unshared electron pair
high EN atom
5% of strength of avg covalent bond
strongest intermolecular forces
determines properties of H2O and biological molecules such as proteins
Intermolecular Attractions and Molecular Properties
physical properties of a compound depend on type of bonding
network solid - all atoms are covalently bonded to each other
