Note
0.0(0)
Explore Top Notes Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 70 people
Studied by 70 people Note
Note Studied by 15 people
Studied by 15 people Note
Note Studied by 47 people
Studied by 47 people Note
Note Studied by 4 people
Studied by 4 people Note
Note Studied by 61 people
Studied by 61 people
GCSE Biology: Homeostasis and Hormonal Coordination
5.0(1)
Chapter 8 - Pakistan Movement in the years 1927-1939
5.0(1)
Microbiology Quiz 6 (BIO 210)
5.0(1)
VCE Biology Unit 3&4
4.5(2)
3. The Self-Strengthening Reforms (1902-1911)
5.0(1)
Ch 24 - Free Trade and Protectionism
5.0(1)
1.3C Electron Configurations of Atoms and Ions
Electron Configuration with Noble Gas Shorthand
Give the electron configuration of the following noble gases
He (2e-) = 1s²
Ne (10e-) = 1s² 2s² 2p^6
Ar (18e) = 1s² 2s² 2p^6 3s² 3p^6
Electrons are lost from the 4s sublevel first
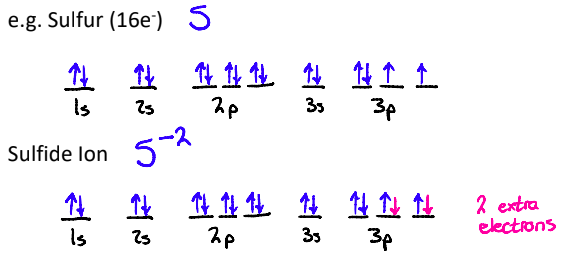
1. Negative Ions
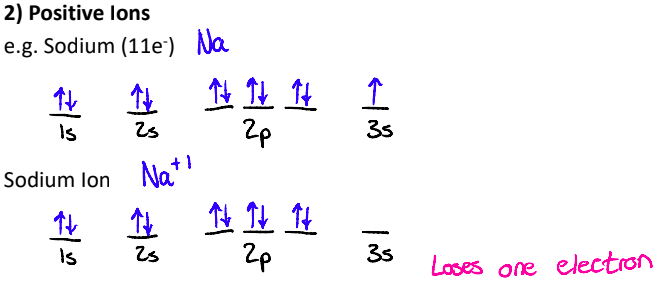
2. Positive Ions
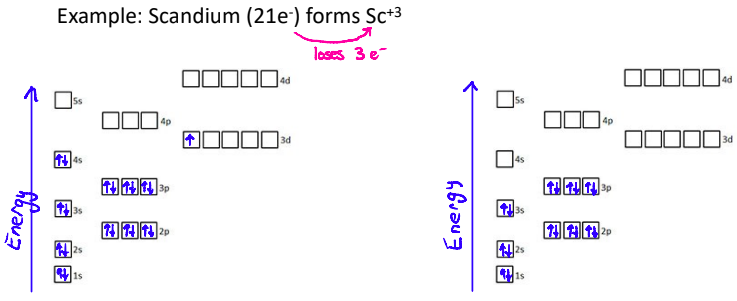
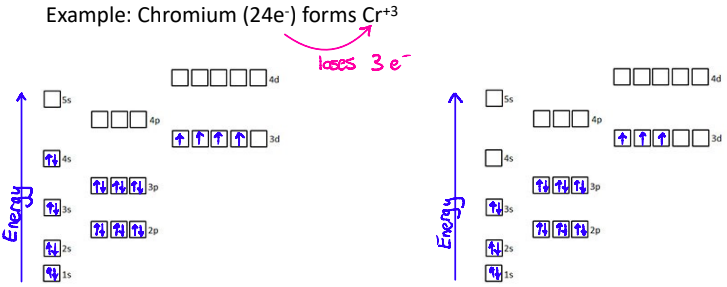
3. Transition Metals
Transition metal ions don’t always follow the pattern of taking on the electron configuration of the closest noble gas
Transition metal electron configurations must be determined from the charge on the ion
Note
0.0(0)
Explore Top Notes Note
Note Studied by 3 people
Studied by 3 people Note
Note Studied by 70 people
Studied by 70 people Note
Note Studied by 15 people
Studied by 15 people Note
Note Studied by 47 people
Studied by 47 people Note
Note Studied by 4 people
Studied by 4 people Note
Note Studied by 61 people
Studied by 61 people
GCSE Biology: Homeostasis and Hormonal Coordination
5.0(1)
Chapter 8 - Pakistan Movement in the years 1927-1939
5.0(1)
Microbiology Quiz 6 (BIO 210)
5.0(1)
VCE Biology Unit 3&4
4.5(2)
3. The Self-Strengthening Reforms (1902-1911)
5.0(1)
Ch 24 - Free Trade and Protectionism
5.0(1)