14 Nitrogen & Fertilizers
Fertilizers and Ammonia
N, K, P fertilizers
Fertilisers contain nitrogen, potassium and phosphorus
- Nitrogen makes chlorophyll and protein and promotes healthy leaves
- Potassium promotes growth and healthy fruit and flowers
- Phosphorus promotes healthy roots
Fertiliser compounds contain the following water-soluble ions:
- Ammonium ions, NH4+ and nitrate ions, NO3-, are sources of soluble nitrogen
- Phosphate ions, PO43- are a source of soluble phosphorus
- Most common potassium compounds dissolve in water to produce potassium ions, K+
Common fertiliser compounds include:
- Ammonium nitrate, NH4NO3
- Ammonium phosphate, (NH4)3PO4
- Potassium sulfate, K2SO4
Different fertilisers contain different amounts of fertiliser compounds so each contain different proportions of nitrogen, potassium and phosphorous


Displacement of Ammonia
- Ammonia can be displaced from its salts by the addition of an alkali substance
- Farmers regularly add basic substances such as calciumhydroxide to their soil to neutralise any excess soil acidity
- If too much of the basic substance is added or if it has been added too soon after fertiliser has been added, then an ammonia displacement reaction may occur
- This involves the loss of nitrogen from the fertiliser, nullifying its effectiveness as a fertiliser
- For example, the salt ammonium chloride is used extensively in fertilisers and reacts with calcium hydroxide:
- 2NH4Cl + Ca(OH)2 → CaCl2 + 2NH3 + 2H2O
Manufacture of Ammonia
Ammonia is manufactured using The Haber process which occurs in five stages:
Stage 1: H2 and N2 are obtained from natural gas and the air respectively and are pumped into the compressor through pipe
Stage 2: The gases are compressed to about 200 atmospheres inside the compressor
Stage 3: The pressurised gases are pumped into a tank containing layers of catalytic iron beds at a temperature of 450 °C. Some of the hydrogen and nitrogen react to form ammonia:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Stage 4: Unreacted H2 and N2 and the product ammonia pass into a cooling tank. The ammonia is liquefied and removed to pressurised storage vessels
Stage 5: The unreacted H2 and N2 gases are recycled back into the system and start over again

Conditions
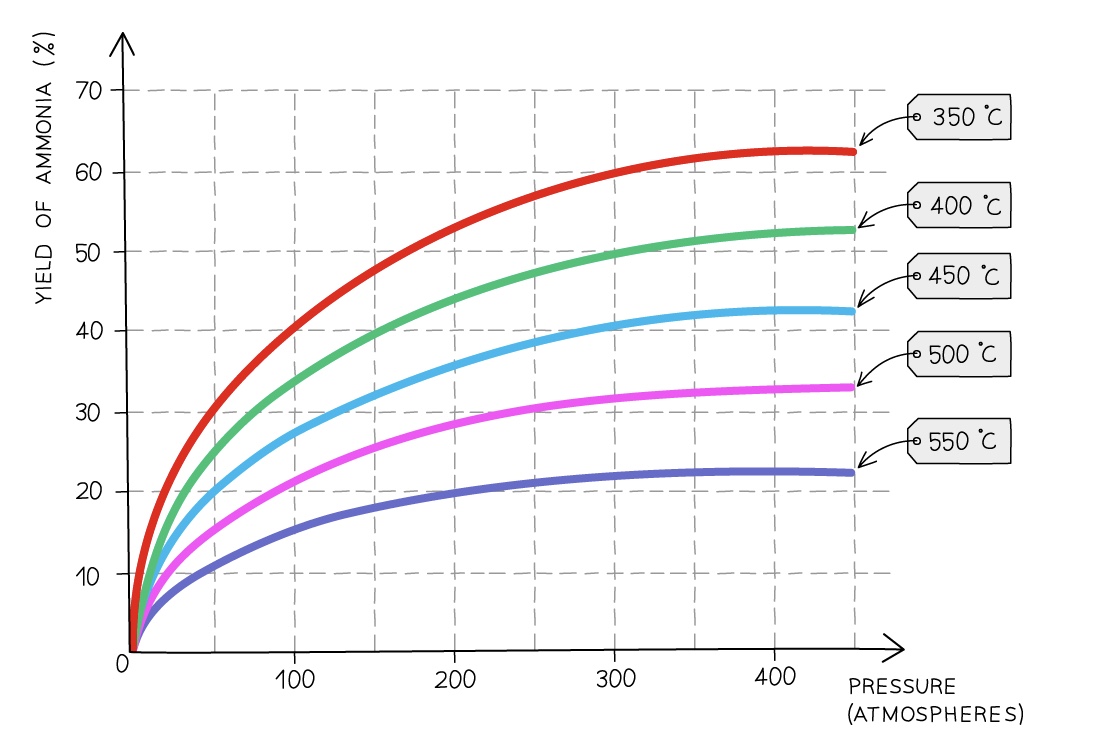
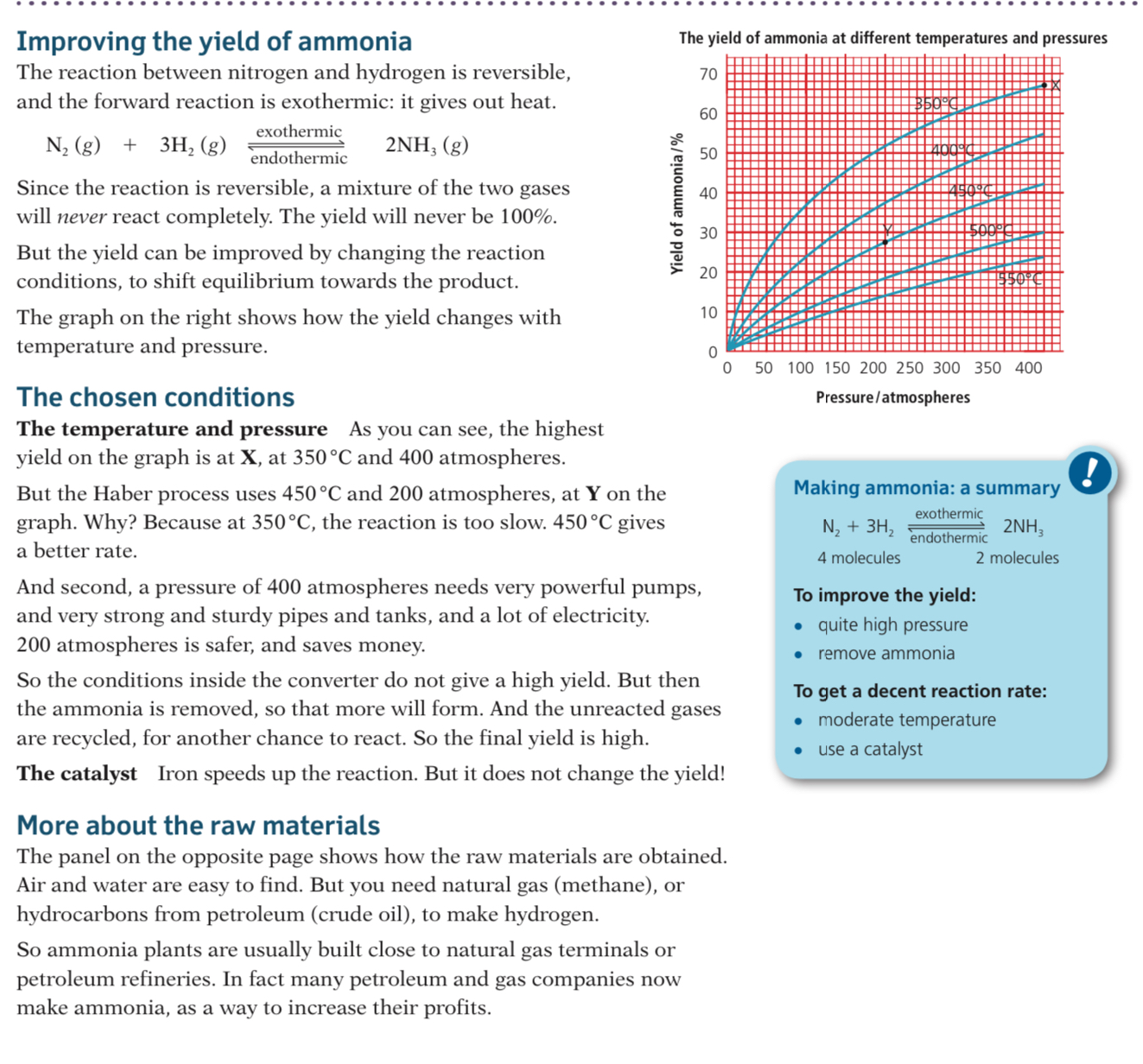
- As the reaction is reversible and the forward reaction is exothermic, the amount of ammonia produced (the yield) can be favoured by altering the conditions
%%Temperature: 450 ºC%%
- A higher temperature would favour the reverse reaction as it is endothermic (takes in heat) so a higher yield of reactants would be made
- If a lower temperature is used it favours the forward reaction as it is exothermic (releases heat) so a higher yield of products will be made
- However at a lower temperature the rate of reaction is very slow
- So 450 ºC is a compromise temperature between having a lower yield of products but being made more quickly
Pressure: 200 atm
- A lower pressure would favour the reverse reaction as the system will try to increase the pressure by creating more molecules (4 molecules of gaseous reactants) so a higher yield of reactants will be made
- A ==higher== ==pressure would favour the forward reaction as it will try to decrease the pressure by creating less molecules (2 molecules of gaseous products) so a higher yield of products will be made==
- However high pressures can be dangerous and very expensive equipment is needed
- So 200 atm is a compromise pressure between a lower yield of products being made safely and economically
@@Catalyst:@@ @@Iron@@
The iron catalyst does not increase the yield of ammonia produced but it does increase the rate of reaction, meaning that more ammonia can be made in a given time