Ionization Energy, Wavelengths,
Where are electrons located in electrons?
Nucleus: Contains all protons (p+) and neutrons (0)
Electrons (e-) are outside the nucleus, dispersed throughout the atom
Electromagnetic Radiation: a stream of particles, called photons, that travel as a cone
To find energy, given frequency: Energy = h * v
To find energy, given wavelength: Energy = (h*c)/λ
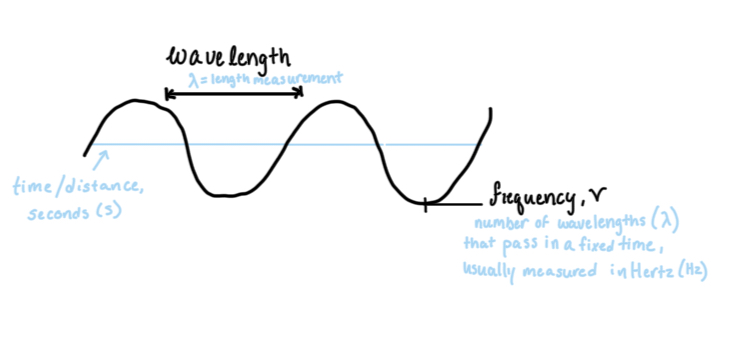
λ: wavelength (lambda)
v: frequency
h: Planck’s constant, 6.626×10^-34 joules-seconds
c: speed of light, 2.998×10^8 meters/second
As frequency goes up: energy goes up, directly proportional relationship
As wavelength goes down: frequency goes up, inverse relationship
To find wavelength, given frequency: λ = c/v
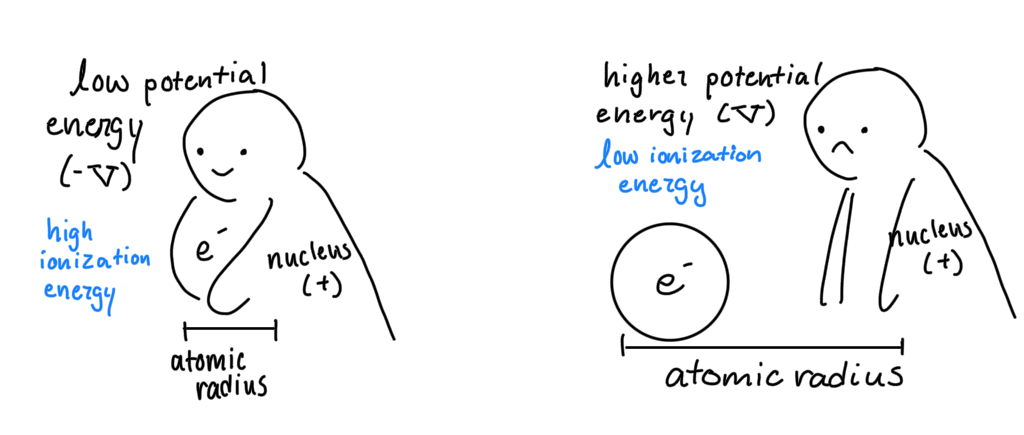
Potential Energy: stored energy an electron (-) has because of how far or close it is the nucleus (+).
Coulomb’s Law: like charges (e- and e-) repel from each other, and opposite charges attract (e- and p+); particles closer together experience more repulsion/attraction, and inverse for particles farther apart
Potential energy is the force of which electrons are being acted upon. So positive potential energy particles are not being as tightly to the nucleus as a negative charge
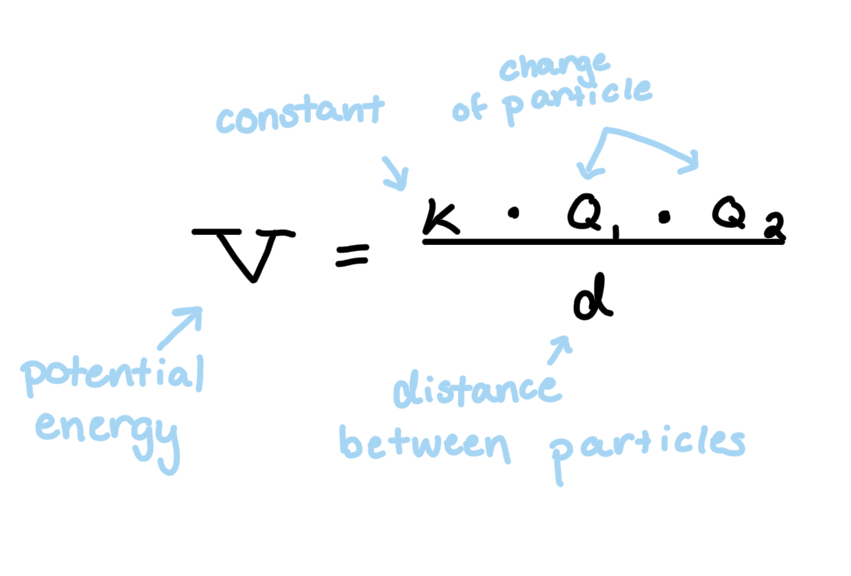
Note: dividing by a larger number makes the entire value smaller, like ¼ is smaller than ½
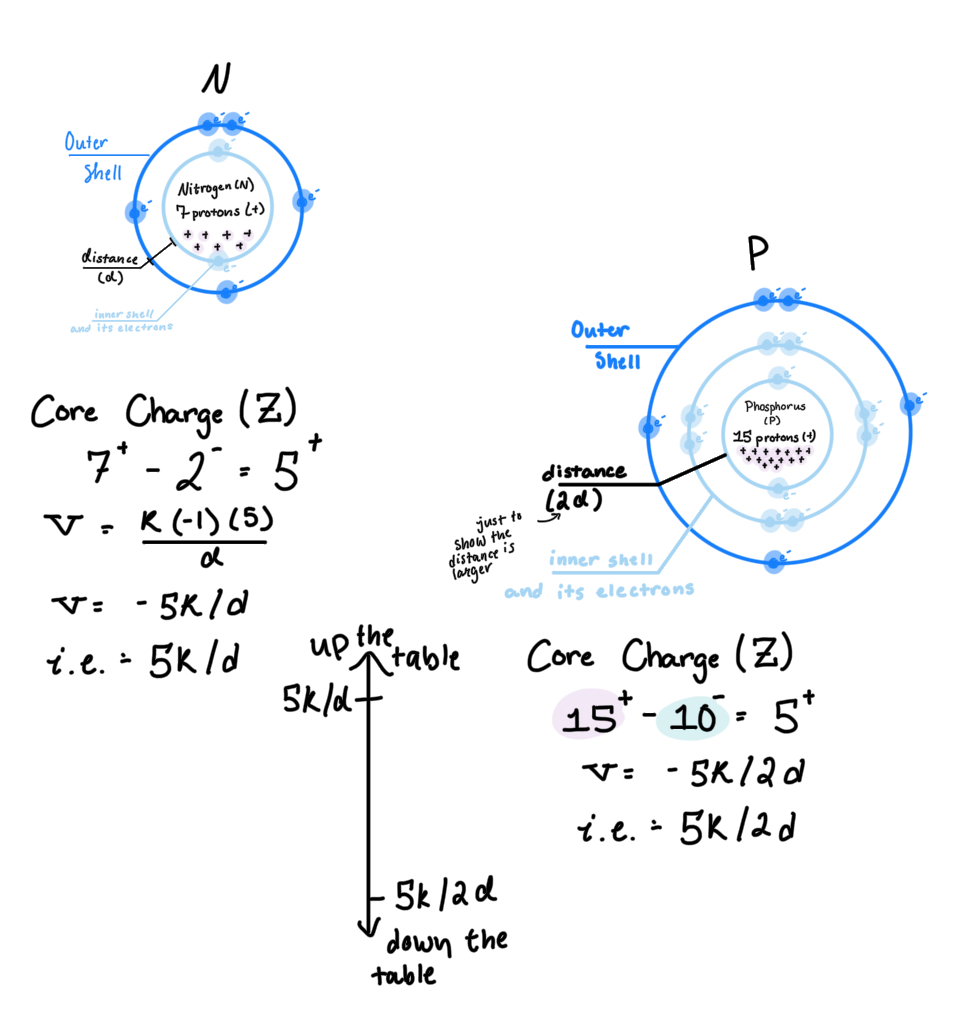
Ionization Energy: the amount of energy needed to remove an electron from an atom in the gas phase. In other words, the energy needed to overcome the attraction of molecules calculated w/ Coulomb’s Law (v=-i.e.)
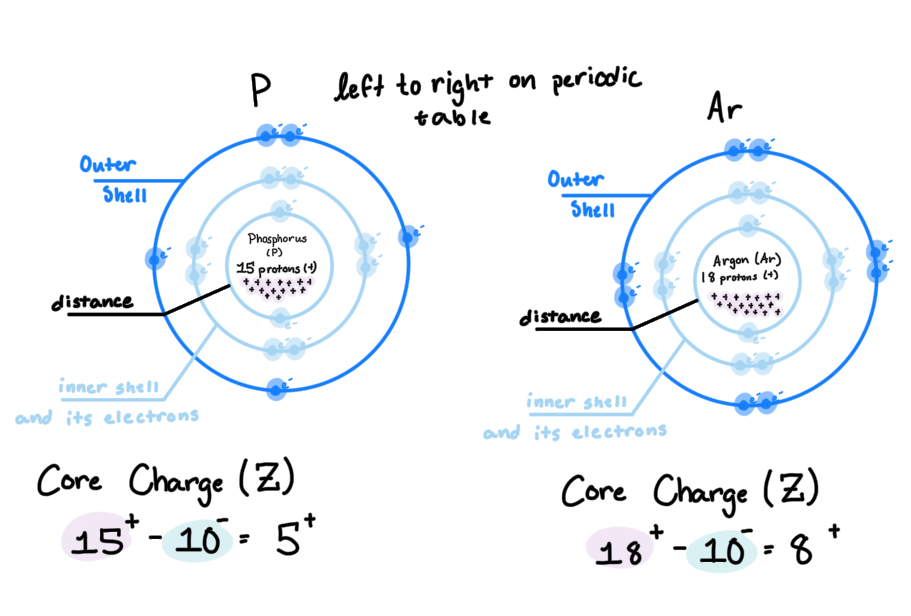
Core Charge: can be used as Q2 in Coulombs law, and is calculated by combining the number of protons in the nucleus (+) and the inner shell electrons/core electrons
Trends in Ionization Energy: as you move to the right, ionization increases. as you move up a column, ionization increases (pictured above)
Relationship between Potential Energy and Ionization Energy: because v = -i.e., that means as potential energy increases, ionization energy decreases
Ionization Energy/Potential Energy and Atomic Radius: I.E. is the amount of energy needed to remove an electron from an atom. So if an electron is far from its center (nucleus), it won’t take much energy to remove. Thus, lower i.e. (and higher potential energy) means a larger atom.
So, if lower i.e. means a larger atom, and i.e. lowers as you move to the left of the table (and down), then as you move left and down the table, the atom increases in size (atomic radii and atomic size)
Established trends in the periodic table, so far:
Electrons are stored in “shells” or energy levels
The first shell is 1. It holds 2 electrons
The second shell is 2. It holds 8 electrons.
Ml can be -l to +l
Ms + charge of the spin of the electron, -1/2 and +1/2