Chapter 17 - The Cytoskeleton
The cytoskeleton is comprised on many smaller parts, small soluble subunits for large filamentous polymers. The formation/deformation of these can be signal, for instance by a nutrient source. It may cause the disassembly of filaments into subunits that can diffuse, or it may signal the reassembly of filaments at a new site. This allows it to be highly dynamic
There is actin, intermediate filaments, and microtubules. Actin stretches all throughout the cell, going underneath the nucleus. Microtubules go from the cell exterior, and intermediate filaments are more concentrated towards the nucleus. Actin tends to be right under the plasma membrane, and supporting structures such as microvilli. Intermediate filaments are “steel cables”. Microtubules form hollow cylinders.
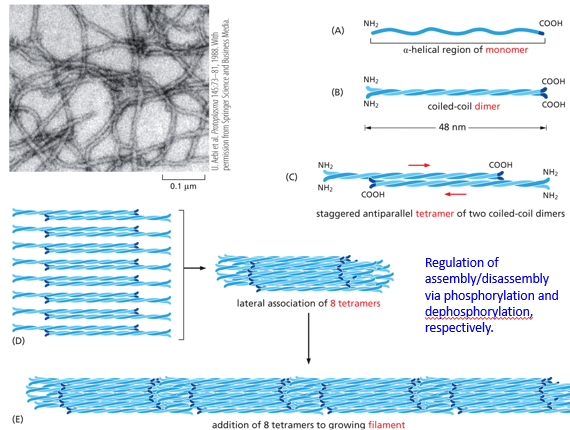
Intermediate filaments (IF) lack polarity. They are comprised of an alpha-helical monomer that will form a coiled-coil dimer with another, which will then stagger antiparallel with another to form a tetramer of two coiled coil dimers. This antiparallel nature negates the polarity of the dimers. 8 tetramers laterally associate, and then get added end to end. Tetramers are the “monomers” or intermediate filaments, when the whole structure disassembles tetramers are left alone. This assembly process and disassembly process occurs via phosphorylation and dephosphorylation. Keratin is an IF, used in epithelial cells cytoplasmically
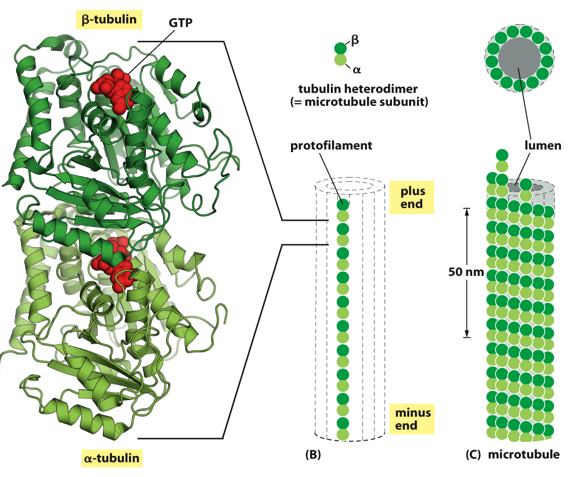
Microtubules have a polarity to them. A monomer is tubulin, which will for a heterodimer protofilament, with a positive and negative end. Tubulins can be alpha, beta, or gamma. A tubulin dimer has an alpha and a beta tubulin, which is the individual building block for microtubules and give it polarity. These then associate with each other to form a cylinder, known as the microtubule. These are used in the process of cell division, helping pull the cell apart and latch on to chromosomes to pull them into daughter cells.
A GTP will “glue” the dimer of an alpha and beta monomer together. It is only hydrolyzed into GDP when the filament is constructed and destructed.
They only grow/shrink at the + end, in a spiraling fashion rather than a protofilament fashion.
All centrosomes are MTOCs, but not all MTOCs and centrosomes. These are where microtubules form from, - end to + end
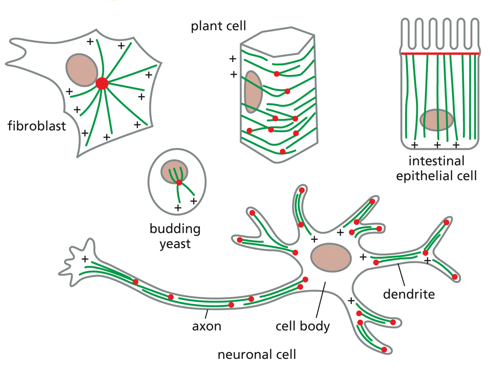
Organization of microtubules is different between cells, each differentiation has a different way of orienting them
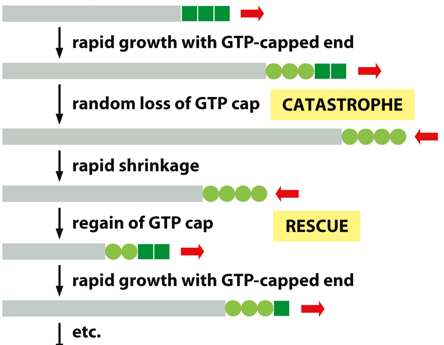
Microtubules exhibit dynamic instability, undergoing rapid growth with the GTP-capped end, random loss of the GTP cap, rapid shrinkage, regain of the GTP cap, and then rapid growth with the GTP-capped end, following with the process repeating. Since it only grows/shrinks at one end, it has dynamic instability. Beta is the + end. Over time, GTP will hydrolyze to GDP (molecular timer), but as long as there are more dimers with GTP still, the polymer will stay together. Disassembly will start once all GTP is hydrolyzed to GTP, and it catches up to the GTP cap.
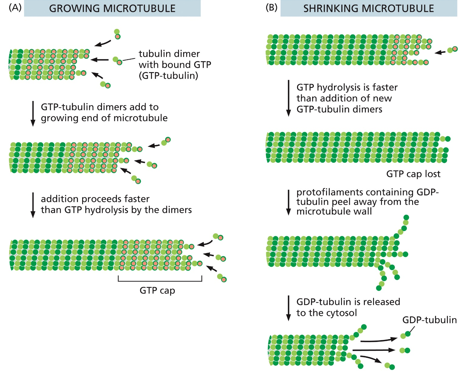
A centrosome is a microtubule organization center (MTOC). They grow from their + ends from a gamma-tubulin ring, with each fiber growing and shrinking independently of one another. The - end is fixed at the MTOC.
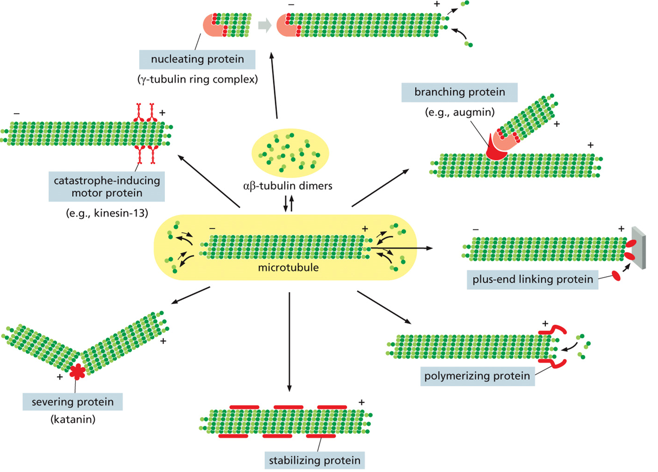
There are many different proteins that associate with the microtubules, such as nucleating proteins, branching proteins, +-end linking proteins, polymerizing proteins, stabilizing proteins, severing proteins, and catastrophe-inducing motor proteins. All of these proteins make the microtubules hard to see, as they are almost nearly fully covered.
The gamma tubulin ring complex is where microtubules nucleate from. This involves controlling the number of microtubules, having a template for their formation, determining microtubule polarity, and controlling the time and location of the assembly. The gamma ring is a single structure with a high affinity for alpha, which is what dimers start joining and spiraling up from.
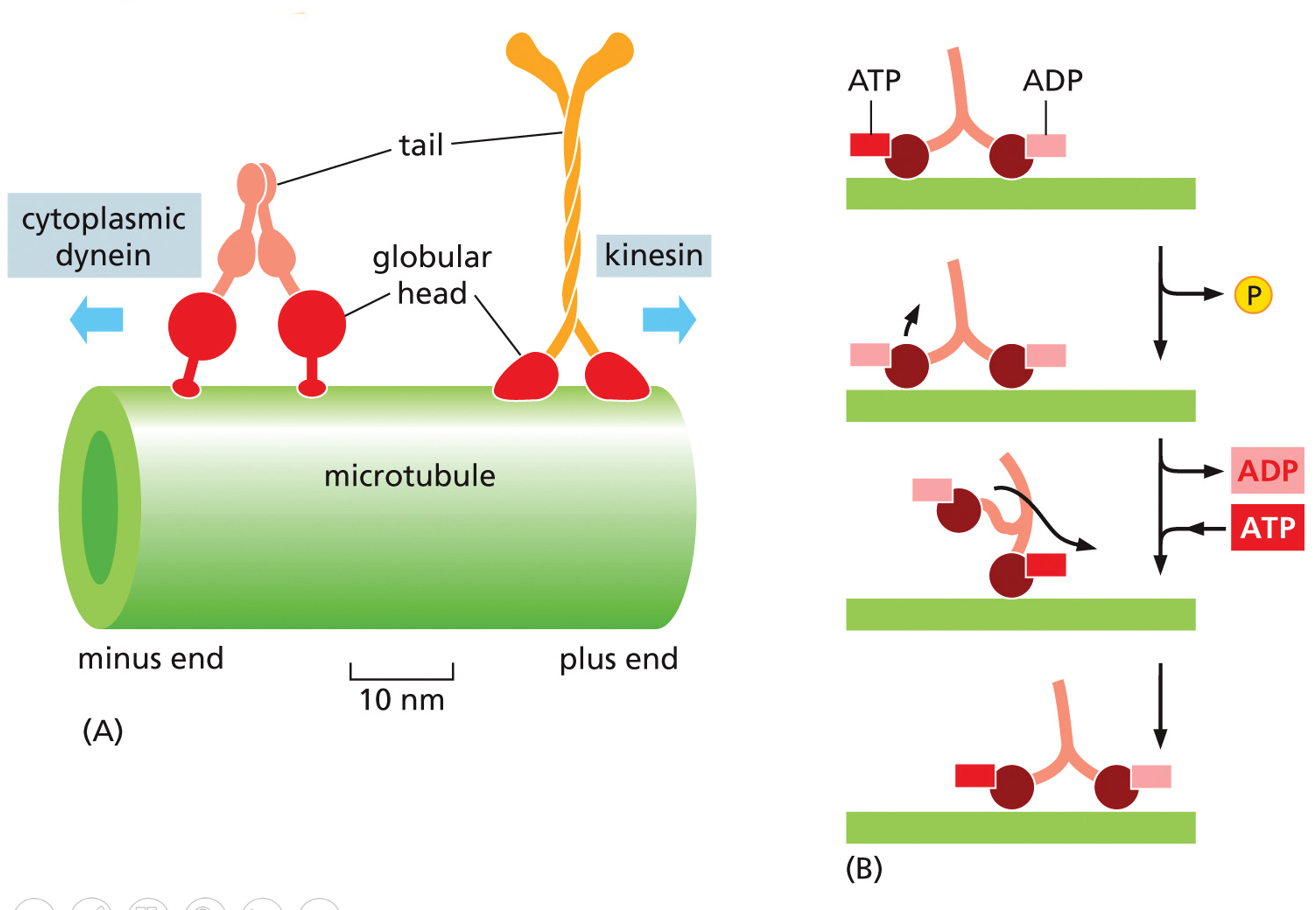
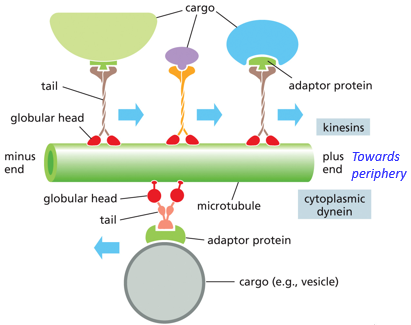
Microtubules are involved in intracellular transport, through the use of motor proteins. Their movement is ATP dependent, through the use of dyneins and kinesins. Kinesins go towards +, and dyneins go towards the MTOC (- end). Dyneins take endosomes to the lysosome, kinesins take vesicles from the golgi to the plasma membrane
Parts of a motor protein include the head domain (cytoskeleton interphase), hinge domain, and tail. The head is also where ATP hydrolysis occurs (ATP → ADP), this part attaches and associates with the microtubules (thing of it like shoes). This energy is what powers the power-stroke (conformational change in the hinge domain).
The tail is very variable. This allows for the motor protein to carry different cargo, it binds to the cargo (vesicles/chromosomes/organelles)
Myosins are also motor proteins that are ATP dependent. Different types of kinesins vary in tails, but they all share the same head domain. They belong to what is called a superfamily.
The positioning of various organelles is determined by microtubule placement and distribution throughout the cell, they “fence them” in place. They also allow for transport to occur between various organelles
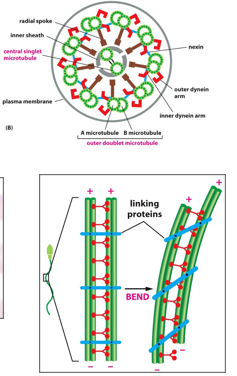
Cilia and flagella also use microtubules
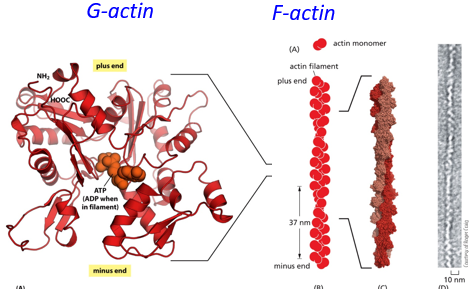
Actin filaments (microfilaments) are also intrinsically polar, with many actin monomers forming a polymer string with a plus and minus end. They can grow/shrink at both ends, unlike microtubules. They are found within microvilli, stress fibers, filopodia, and the contractile ring. They tend to stretch from one end of the cell to the other. G-actin is a free actin monomer, and F-actin is filamentous actin.
The membrane cortex is formed by actin filaments crowding/supporting the exterior of the cell, as they exist under the cell membrane.
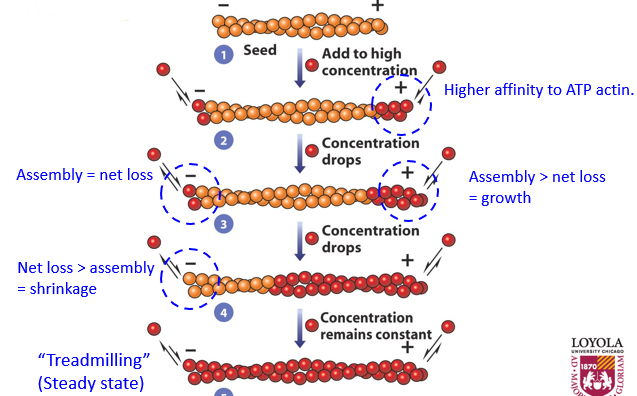
To assemble an actin filament, the process of treadmilling occurs. When there is more assembly than net loss, there is growth. The opposite is shrinkage. When there is a steady state of both, the filament is treadmilling. This whole process is ATP dependent. The + end grows faster than the - end. As the filament grows, there is a lower concentration of G-actin since it is all F-actin, slowing growth and causing the - end to lose monomers at the same rate that the + end grows. The + end will keep growing but at the same state that the - end is losing, this ‘stable’ state is called treadmilling, with a ‘constant’ length. Concentration of G-actin and F-actin remains constant at this point.
Once G-actin gets added to F-actin, after a bit it will hydrolyze ATP to ADP, and will remain part of the filament so long as other monomers have been added to the end.
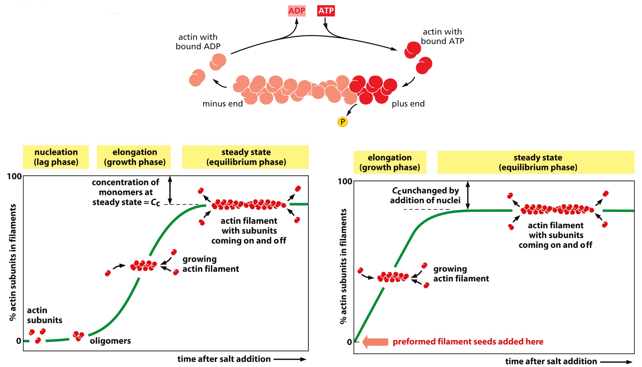
This diagram models G-actin against F-actin, showing concentrations as treadmilling is trying to be achieved. Instead of starting from nothing, the cell will start with “seeds” of F-actin, to start further along on the curve.
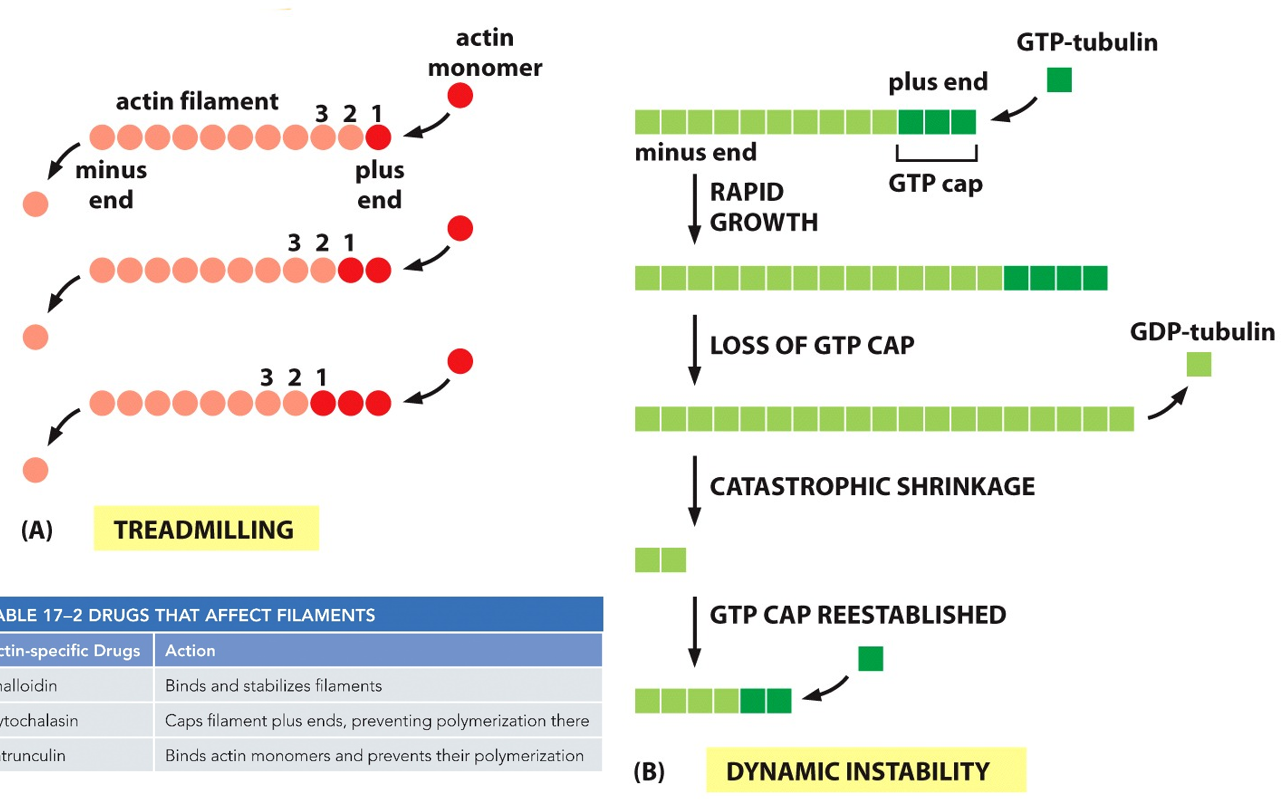
This is the different between dynamic instability and treadmilling, learn this.
There are many actin binding proteins, such as nucleating protein, severing protein, cross-linking protein, capping (+ end blocking) protein, side-binding protein, myosin motor protein, and bundling protein. There is also a monomer sequestering protein.
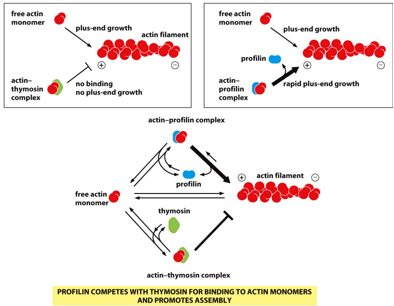
Profilin and thymosin will work together as counterplayers in regulating actin filaments. Profilin bind to actin monomers to increase filament formation. Thymosin binds to the same site on the monomer, and promotes disassembly of filaments. When the cell knows it needs to form/deform polymers, it will use these proteins (for example contractile ring formation in mitosis)
There are also many drugs that can kill the cell by targeting actin, either by stopping polymer formation or stopping filament disassembly.
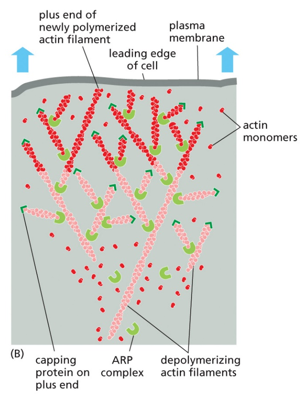
Cellular migration occurs via the use of actin and myosin.
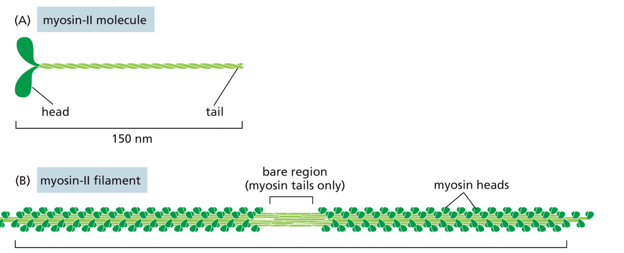
Myosin in a motor-protein than actions on actin. Structurally, it has a head and a tail, with many monomers coming together to form a polymer with many heads associating around many tails bundled. There is a small region where there are only tails, called the bare zone. There are many types of myosin, from I-XIV. There is a highly conserved head motor domain, and a highly divergent coiled coil tail domain in each.
Myosin II functions in muscle contraction, and facilitates cytokinesis. It has a bipolar organization (polarity switch halfway through, mirrored), with thick filaments. It creates leverage to move other filaments relative to each other, and is highly stable. Tails wind around each other for stability in myosin II (called the thick filament or myosin bundle). It always walks towards the + end
Myosin V is like kinesin, it is responsible for endocytosis and exocytosis.
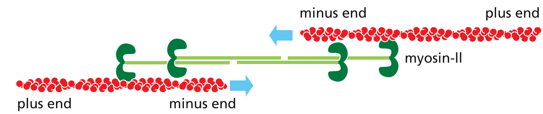
Myosin and actin interact to move muscles, in a process known as power stroking. ATP will bind on the rear head domain, and release actin. This ATP is hydrolyzed, and the phosphate is released. ADP is released, and the power stroke is formed.
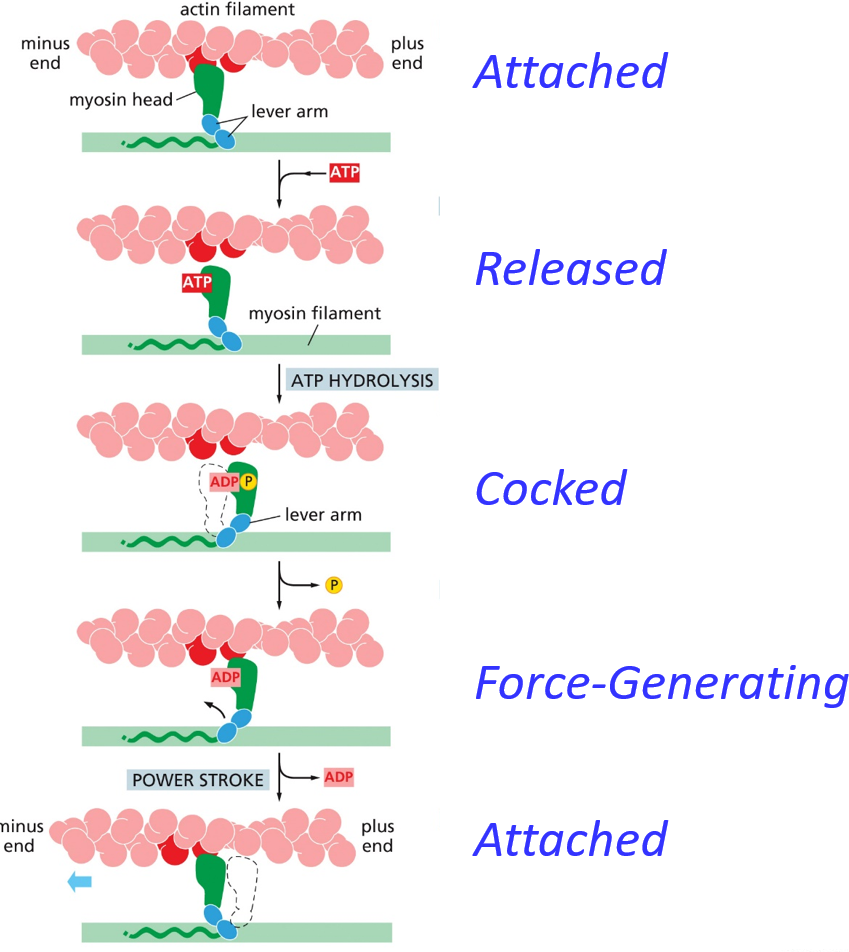
The ‘default’ is a myosin head attached to actin. ATP binds causes a conformational change, releasing the actin head from the myosin filament. The ATP hydrolyzes, and this energy causes a conformational change to load the lever arm (hinge domain). The phosphate is released, and then a new actin head binds to the myosin. This whole process can be related to a rowing crew on a boat. Unlike this analogy, it isn’t coordinated, rather more of a crawling motion.
Skeletal muscles are anchored to bones, and are controlled voluntarily. They have extremely large multinucleated cells (made from fusion of myoblasts). They are made of muscle fiber, and have nuclei on the periphery. They have characteristic banding patterns, and subcellular units of myofibrils. The contractile unit is the sarcomere. The muscle is comprised of muscle bundles which each are surrounded by a muscle sheath, each bundle has many muscle fibers, comprised of multinucleated cells, which each have many myofibrils.
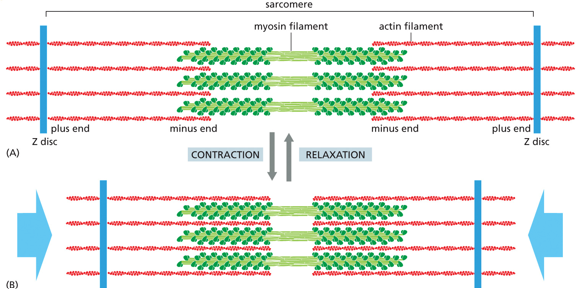
Actin and myosin interact under the sliding filament model to produce skeletal muscle movement. This involves both contraction and relaxation. As myosin ‘walks’ the white band gets smaller but the dark band doesn’t change, the myosin walks more towards the Z-disc, or the positive end of actin.
Each sarcomere is from Z-line to z-line. There are light bands on either side of the Z-disc, and dark bands (myosin filaments) where polarity switches. Halfway through the myosin filament is the M-line. The distance between the - ends of actin is the H-zone, which shrinks during contraction.
Actin filaments are fixed, which means that there is no treadmilling
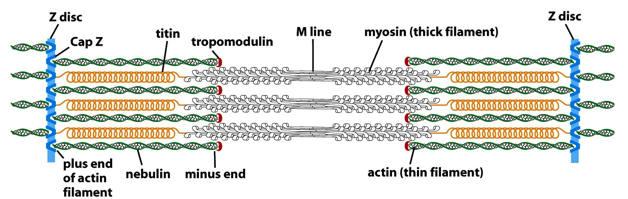
Since actin filaments must stay the same length, there are capping proteins on either end. The + end has Cap Z (which forms the Z-line). On the - end, there is tropomodulin. This guarantees all actin is the same length and stays this way. Nebulin is a stabilizing protein, stabilizing the length portion or actin. Myosin, as a thick filament, doesn’t require as much stabilization from proteins. There are titins, which are spring like proteins that position the myosin. Titin also helps in muscle relaxation.
The sarcoplasmic reticulum consists of transverse tubules formed from invaginations of the plasma membrane. These are tunnels from one side of the cell to the other, allowing for extracellular material to flow through. Ca2+ release channels are located in the membrane of this, and the release of these allows for the sarcoplasmic reticulum to open. The opening of the voltage gated channel allows for the opening of the ligand-gated channel on the sarcoplasmic reticulum. There are also many calcium pumps, moving Ca2+ back into the sarcoplasmic retilulm and out of the cytosol.
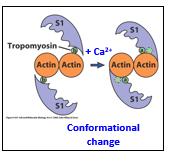
Troponin is an actin binding protein. These build the troponin/tropomyosin complex. In relaxed muscle states, this prevents myosin from binding with actin. Calcium enters and binds to this complex, causing a conformational change and moving tropomyosin out of the way, allowing actin and myosin to bind and start moving together. S1 is the head domain of the myosin.
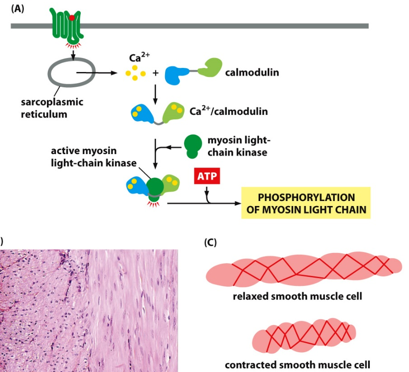
There is also contraction in smooth muscle cells. This is involuntary.
Myosin V is a kind of myosin that walks to the + end, functions like a motor protein. It is analogous to kinesins.