Chapter 15 - Intracellular Compartments
There are many different organelles within a cell, membranes allow for these to have different environments, through a process known as compartmentalization. Examples of these organelles include the nucleus, lysosomes, peroxisomes, RER, and mitochondria
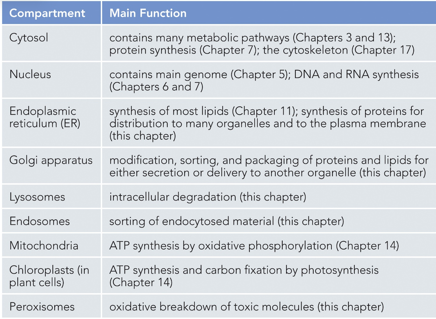
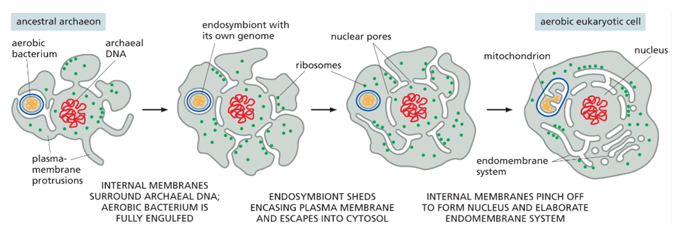
Evolutionarily, an aerobic bacterium must have been engulfed by an anaerobic one (endosymbiont theory), each with their own genomes. The endosymbiont must have shed its plasma membrane into the cytosol of the larger bacterium, and internal membranes must have pinched off to form other membranes like the nuclear membrane. Eukaryotes have an endomembrane system, prokaryotes do not.
There is compartmental infrastructure, with a cargo molecule being transported. Many organelles transport vesicles between each other, and with the plasma membrane (extracellular environment).
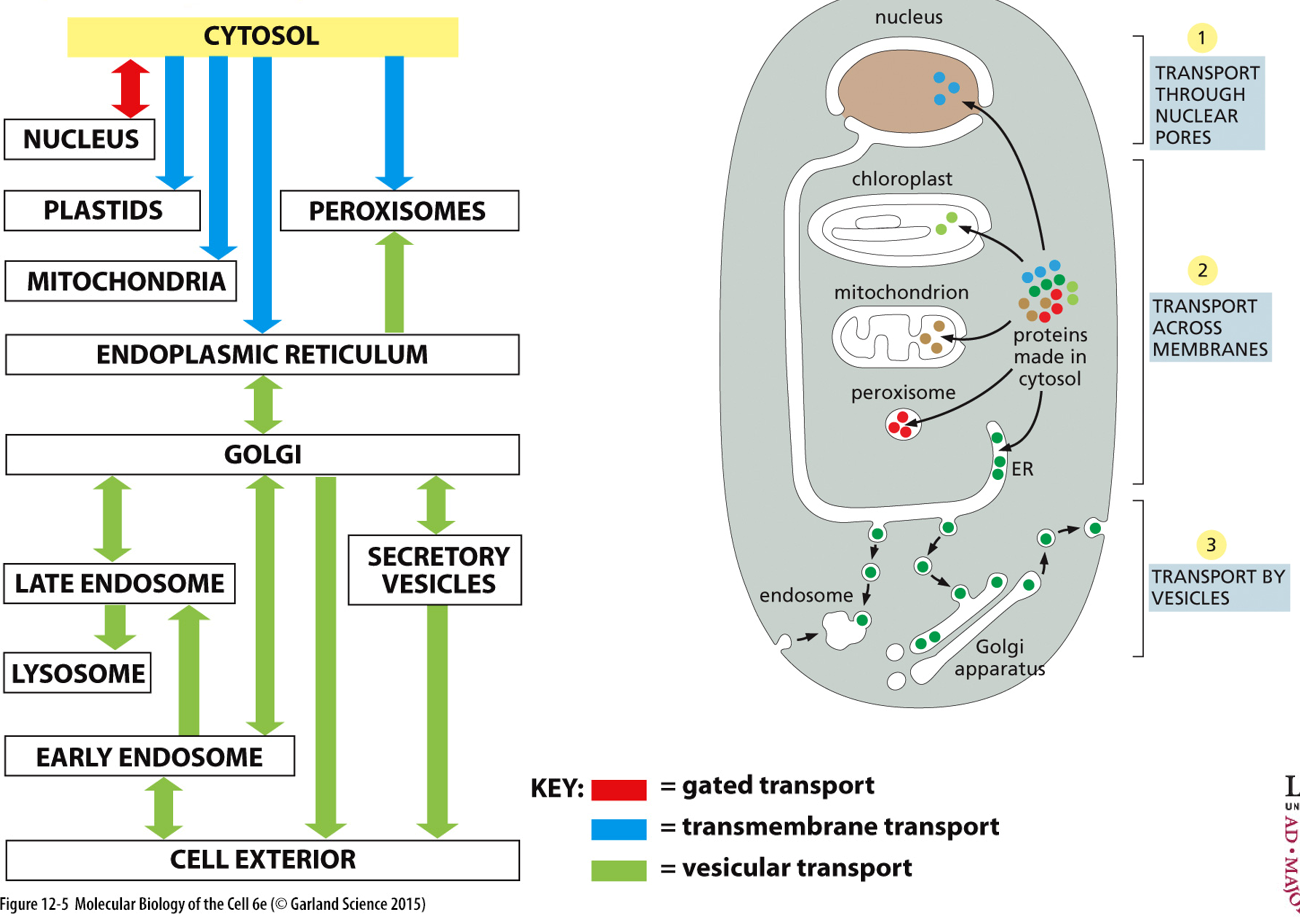
Gated transport occurs between the nucleus and the cytosol, transmembrane transport between cytosol and plastids/mitochondria/ER/peroxisomes, and vesicular transport for everything else.
When a protein is made, it either remains in the cytosol, or it doesn’t. Example glycolysis enzymes will stay, while CAC proteins do not (mitochondrial functioning). Collagen is for extracellular structure, so it doesn’t.
To transfer a protein to the nucleus, the protein will travel through the nuclear pores, through the use of a specific sequence. Examples are histones and polymerases.
Transport of membranes is also a form of protein transport, for proteins that end up in organelles with membranes (like the mitochondria, peroxisome, etc.) Translocators (translocons) are proteins that can help transport other proteins across a membrane. These translocons are on the membranes and allow in the proteins
Vesicular transport is for all the proteins that need to go into the plasma membrane, or out of the cell, or into the lysosome, or the golgi apparatus. These will initially go into the ER (via transport across membranes) and then into a vesicle which can undergo endocytosis/exocytosis.
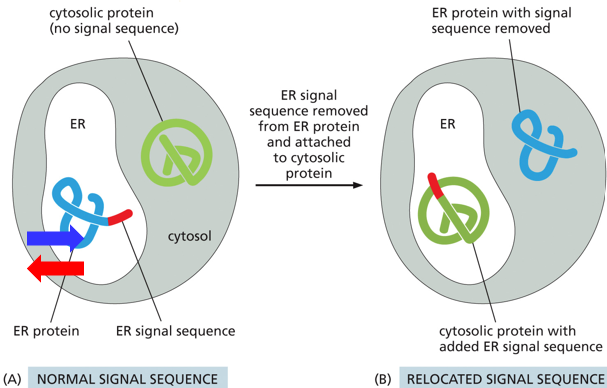
For proteins not staying in the cytosol, they have a signal peptide sequence which indicates their intended location. This signal sequence is part of the protein’s sequence (and DNA sequence as well). A protein supposed to go to the ER will have an endoplasmic reticulum signaling sequence. The protein itself and its function is irrelevant to the process, as demonstrated experimentally by adding a signaling sequence to a protein not intended to go to the ER and seeing it go into the ER.
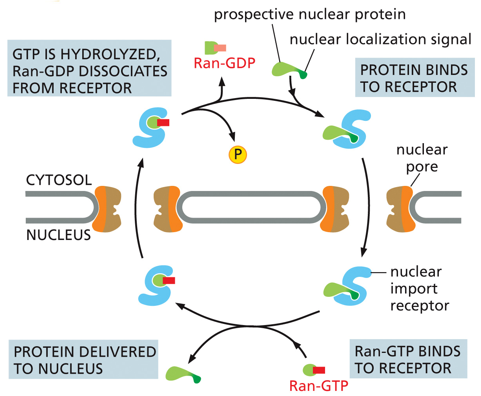
Gated transport allows for nuclear localization signals (NLSs) within the nuclear pores. GTP will be hydrolyzed on the nuclear membrane, releasing Ran-GDP and a phosphate. A protein trying to enter the nucleus will have an NLS attached and bind to a receptor on the nuclear pore, and once inside the nucleus the protein will be delivered and replaced by Ran-GDP, and then this will be hydrolyzed and released in a loop. The protein has both an import and an export sequence. (Know concept, not in depth)
The endoplasmic reticulum is the organelle that allows proteins the enter vesicular transport. It sends everything through the golgi apparatus, which then has diverting pathways
The Smooth Endoplasmic Reticulum (SER) is extensively developed within skeletal muscles, kidney tubules, and steroid producing endocrine glands. It functions as the synthesis of these steroid hormones, in the detoxification of the liver, and in sequestering Ca2+ in skeletal/cardiac muscle cells (the sarcoplasmatic reticulum)
The Rough Endoplasmic Reticulum (RER) is the site of synthesis for proteins, carbohydrate chains, and phospholipids with specific destinations
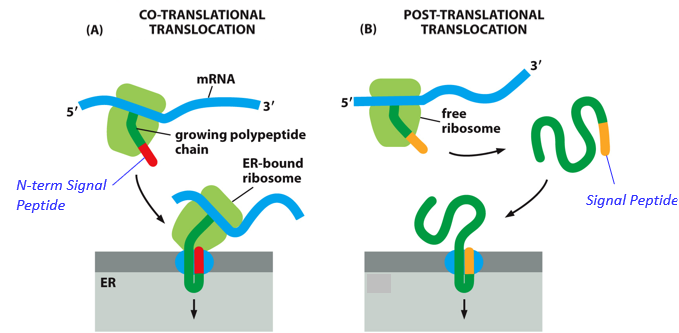
With Cotranslational translocation, proteins are brought into the ER as they are being synthesized, due to a signal peptide
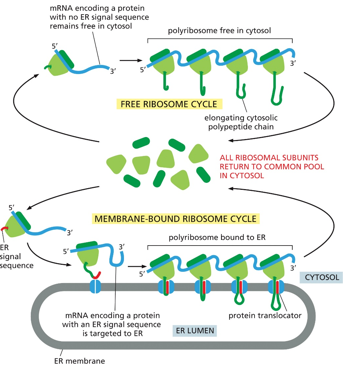
Whether or not a ribosome localizes itself to the ER membrane depends on the presence of a signal peptide in the protein that is translates. With no peptide, it will remain in the cytosol. If there is the signal, the ribosome will locate itself to the ER, where the protein will be translated into the ER lumen. The subunits will dissociate back into the cytosol once translation is done
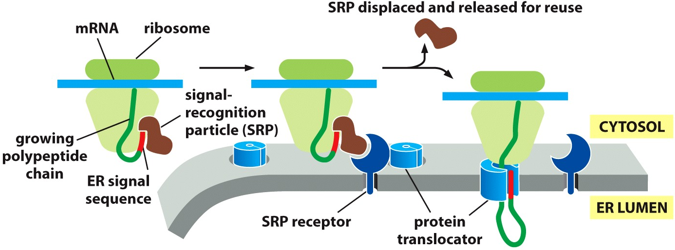
There are signal recognition particles (SRP) that work to bring ribosomes to the ER, through the process of co-translational translocation. The SRP will stop translation, and guide the protein to the ER, where a protein will bind to an SRP receptor (which only recognizes an SRP ribosome complex) and guide the translated sequence into the protein translocator, feeding the protein into the ER. The SRP will dissociate and bind to another signal sequence, and repeat the process. (Kind of like the sigma factor with DNA polymerases)
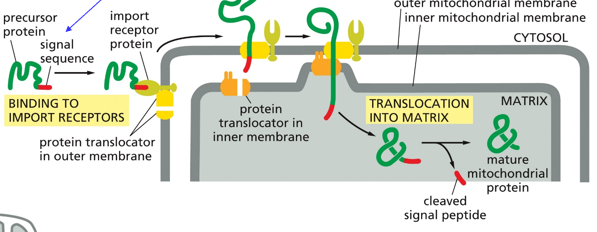
There is also post-translational translocation, or protein transport into the mitochondrion. This requires mitochondrial targeting (signal) sequence (peptide). Proteins on either side of the mitochondrial membrane will work together to get proteins into the matrix.
Within the ER, protein glycosylation will begin. This is when an N-linked bond is formed between an amino acid and a sugar. Sugars like N-acetylglucosamine, Mannose, and glucose are often involved. An oligosaccharyl transferase will help link the sugar tree with the protein
Vesicular transport utilizes the endo-membrane system, with things like endocytosis and exocytosis. This happens between the plasma membrane, lysosome, golgi apparatus, ER, nuclear envelope, endosomes, and transport vesicles.
With vesicular transport, cargo molecules are the ones being transported. They may form via budding and join another region via fusion cycles. They travel from donor compartment to target compartment
The biosynthetic secretory pathway transports proteins out of the cell. The endocytic pathway starts in the plasma membrane and takes things into the cell.
Within the golgi apparatus, a protein will have a sorting signal to designate where it’s final destination is. For proteins that are secreted out of the cell, there is no sorting signal.
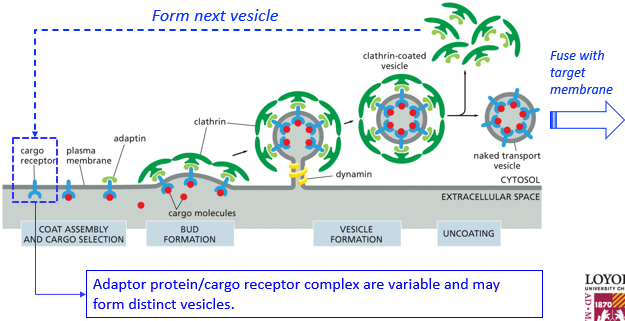
A cargo receptor on the plasma membrane may be bound by various substances including the cargo, which can initiate various processes of vesicle formation and eventual various processes of fusion with target membranes. The molecules to form vesicles may be reused as well. Once these proteins coat a vesicle and budding is complete, the coating proteins will disassociate and be reused. Due to their rigid shapes, they will push against each other and push up the membrane to create the vesicle. A dynamin is a “choking protein” and will bring different ends of the membrane so close that they spontaneously fuse, which finishes vesicle formation.
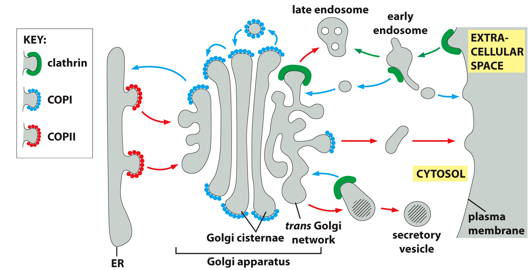
Examples of coating molecules include clathrin, COPI, and COPII. Different functions/areas have different coating proteins. Clathrin is used for the peripheral part of the cell. COPII is directional, helping vesicles from the ER go to the golgi. COPI is for retrograde transport, bringing things into the cell.
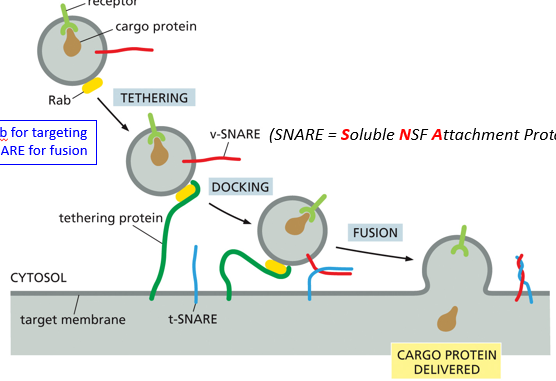
During the process of fusion, Rab will target the vesicle, and SNARE (Soluble NSF Attachment protein REceptor) will aid in fusion. The vesicle is tethered, docked, and then fused. A tethering protein will recognize Rab, and cause a conformational change to pull it in until the snares can interact. Snare interaction will cause the fusion of membranes, and ultimately release the cargo into the target membrane
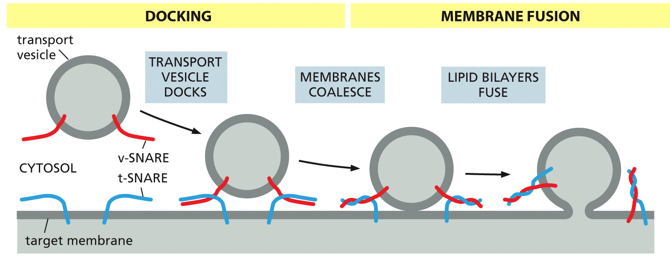
For vesicle fusion, a v-SNARE on the target membrane will intertwine with a t-SNARE on the transport vesicle. Docking is when they first interact, and they twist during membrane coalescence, bringing the vesicle much closer until the lipid bilayers fuse with one another. In nerve cells, this process utilizes Ca2+ as a cofactor, with synaptotagmin which will allow SNAREs to zipper and ultimately fuse. Structurally, snares are alpha helices in dimers or trimers
The Unfolded Protein Response (UPR) helps restore normal function of the cell, degrades misfolded proteins, activates signaling pathways that lead to increased production of chaperones to help these proteins fold, and within a certain time span if not executed can signal apoptosis. Within the ER, a properly folded protein may bud and a misfolded protein may interact with a chaperone.
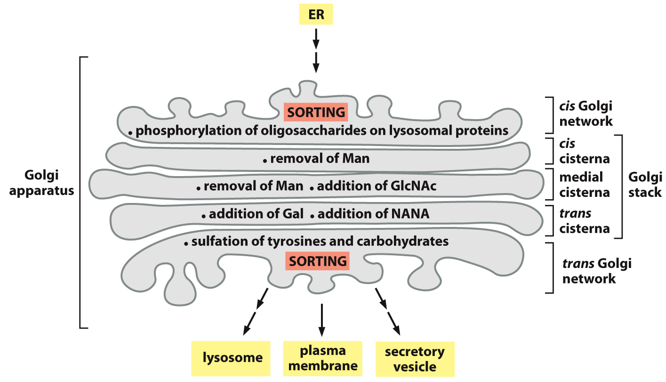
The golgi apparatus helps with oligosaccharide processing. The stack has many different layers, with the top and bottom for sorting. It is involved in the biosynthetic secretory pathway. It is polar and unidirectional. Things enter the top cis part, go through the medial stack, and then gets distributed to final destinations via the trans network which sorts. In the golgi there are many proteins, and lots of post-translational modifications occur here. In the medial stacks, sorting signals can be added. For something to go into the golgi, it needs a signal peptide. Things without signal peptides remain in the cytosol, things without sorting signals get excreted.
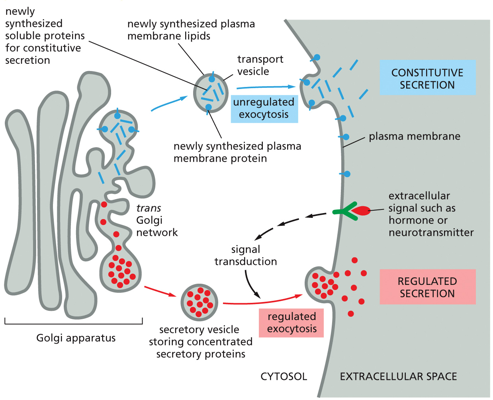
With exocytosis, there is a split between constitutive and regulated secretion. Constitutive secretion involves newly synthesized soluble proteins entering a transport vesicle, and undergoing unregulated exocytosis. This occurs all the time, and is signal independent. With regulated secretion, a transport vesicle will contain concentrated secretary proteins, and will only fuse and undergo exocytosis via an extracellular signal.
The synthesis of the lipid bilayer can vary. For the ER, phospholipid synthesis will add to the cytosolic leaflet. Scramblase will catalyze the flipping of the phospholipid bilayer (every-other phospholipid gets flipped), leading to symmetric growth of both halves of the bilayer as opposed to curvature. In the plasma membrane, due to the asymmetric nature, new portions of membrane will be delivered via exocytosis, and flippase will catalyze the flipping of specific phospholipids to cytoplasmic monolayers
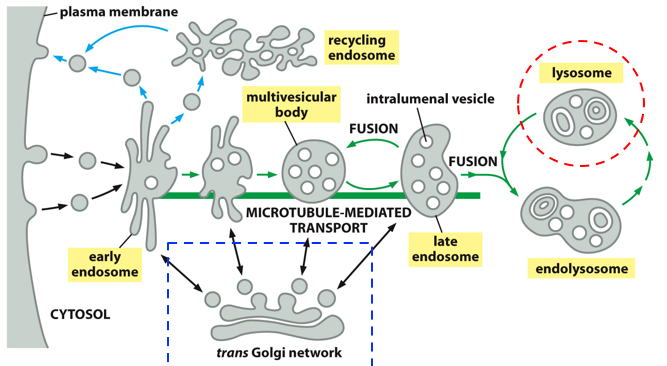
The process of endocytosis is complex. The plasma membrane forms vesicles via budding, forming structures known as endosomes via fusion of these vesicles. The endosome is slowly transported into the cell core, allowing room for another endosome to form. As a late endosome, it fuses with the lysosome forming the endolysosome, where particles are broken down (that were being carried by the vesicles originally), transforming again into a lysosome that can fuse with another late endosome. That whole process is the endocytic pathway (bringing things in for nutrition). Digestive enzymes come from the trans golgi network via the biosynthetic pathway.
Things require a sorting signal to be sent to the lysosome, this signal is specifically mannose-6-phosphate (M6P). This isn’t a signal peptide, as it isn’t a protein.
With the endocytosis of LDL, LDL receptors are activated which initiates clathrin-coated vesicle formation. After uncoating, it fuses with the endosome, where LDL is then delivered to the lysosome. The receptor will undergo budding and return to the plasma membrane. In the lysosome, cholesterol will diffuse out. Once in the lysosome, the cholesterol will be released into the cell, and can cause problems especially in blood vessel cells.
Viruses are taken up via endocytosis, clathrin is the coating protein and they are brought into the cell.
Receptor proteins after being part of an endosome will be recycled through exocytosis, be degraded in the lysosome, or go through transcytosis
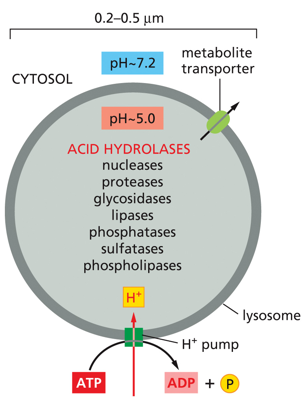
The lysosome has a pH of around 5, and contains many acid hydrolases. Metabolite transporters exist on the membrane, and there are many proton pumps bringing in protons and turning ATP into ADP and Pi. Hydrolases include nucleases, lipases, protases, and glycosylases. These are in the lysosome, along with a B-type proton pump which allows the pH in the lysosome to drop to 5. This allows the enzymes to function. If the lysosome burst and these enzymes were released into the cytosol, they would stop working as the cytosol doesn’t have the pH of 5 that they need. The B-pump increases the concentration of H+.
There are 3 pathways to degradation within the cell, either phagocytosis (bacterial entrance within a membrane), endocytosis, or autophagy (formation of a membrane around part of the cell that is already there)
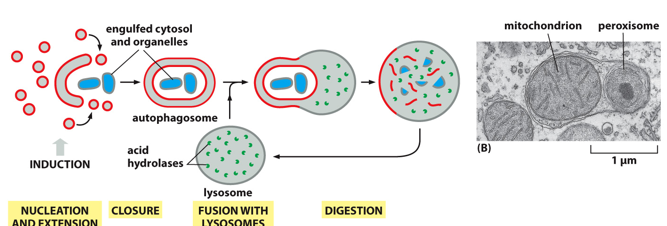
With autophagy, it is done under starvation, stress, and organelle maintenance. It starts with nucleation and extension which forms a membrane, and fills it ending in closure (known as an autophagosome). This membrane will fuse with the lysosome, and the particles inside will be digested. Phagocytosis surrounds a much larger particle like a whole bacteria, and coats it with clathrin internally.