Radioactivity

Radioactive material- material that emits alpha, beta or gamma particles.
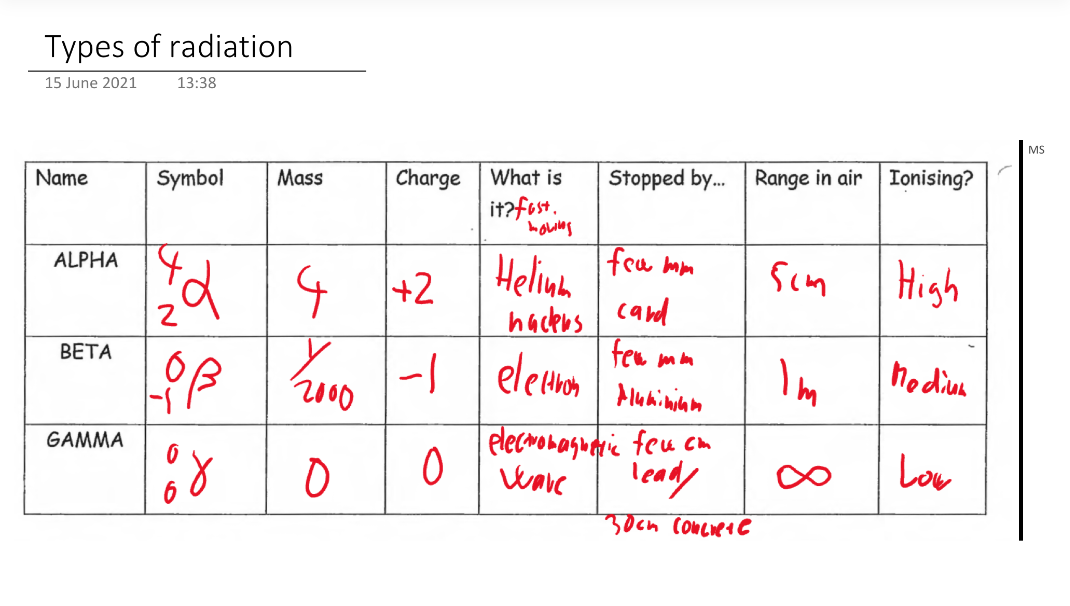
Radiation - (alpha, beta or gamma) is always emitted from an unstable nucleus. Many elements have both stable and unstable isotopes.
Ionising- The radiation can turn atoms into ions by removing (or adding) electrons. This can damage DNA and cause mutations, leading to cancer.
Radioactive decay is random and spontaneous process. Random means we cannot predict exactly which atom will decay when, spontaneous means it is not affected by external conditions (temperature etc).
Radioactive Decay - when unstable nuclei emits alpha, beta or gamma radiation.
Background radiation- It is low level radiation that is always present all around us. Some sources of background radiation are:
cosmic rays,
hospitals/radioactive waste or tracers,
fallout from nuclear test,
Carbon 14 (in carbon dioxide in air),
rocks and soil, Radon gas,
Food
Half life- When radioactive (unstable) nuclei decay, they will eventually turn into stable nuclei. This means radioactive material will lose its activity over time.
Uses of radioactivity- Sterilising food/medical equipment: Equipment blasted with huge amounts of radiation to kill all bacteria/viruses. Gamma so that all parts and sides of the equipment can be reached.
Radiation safety
Worn outside to clothing to detect radiation. When exposed to ionising radiation the film turns black
Alpha - only the top third will turn black
Beta - top 2 thirds will turn black
Gamma - all 3 will turn black
How to minimise exposure to radiation (for those who work with ionising radiation)
Lead lined apron for X-rays : minimise dose to other parts of the body (X-ray)
Stand behind a lead wall (which will absorb radioation)
Increase the distance from the source (radiation intensity decreases dramatically with distance)
Minimise exposure time
Use robots (nuclear power stations)
Using tongs when handling radioactive substances,
Wear gloves/lab coat when there is a risk of contamination, it will not stop beta/gamma, but it will avoid radioactive substances contaminating hands/clothes of the person
Radioactive Material
Definition: Radioactive material refers to substances that emit radiation in the form of alpha, beta, or gamma particles as a result of nuclear reactions occurring within unstable isotopes.
Radiation
Types: Radiation can be classified into three main types: alpha particles (helium nuclei), beta particles (electrons or positrons), and gamma rays (high-energy electromagnetic waves). These emissions are always associated with unstable nuclei, which can lead to various interactions with surrounding matter.
Isotopes: Many elements possess both stable and unstable isotopes, playing a crucial role in their radioactive properties and applications.
Ionizing Radiation
Mechanism: Ionizing radiation is capable of stripping electrons from atoms, producing ions. This ionization process can result in molecular damage, particularly to DNA, which may lead to mutations and the potential development of cancer.
Radioactive Decay
Nature of Decay: Radioactive decay is a random and spontaneous process, meaning it is impossible to predict precisely when a specific atom will undergo decay. This process occurs independently of external influences such as temperature or pressure.
Emissions: During radioactive decay, unstable nuclei transform by emitting alpha, beta, or gamma radiation, leading to the formation of new elements or isotopes, often culminating in stable configurations.
Background Radiation
Definition: Background radiation is the low-level radiation that is omnipresent in our environment, emanating from both natural and artificial sources.
Sources: Key sources of background radiation include:
Cosmic rays from outer space.
Radioactive waste and tracers used in medical facilities.
Fallout from nuclear weapon tests.
Carbon-14 present in carbon dioxide in the atmosphere.
Naturally occurring radioactive materials in rocks, soils, and radon gas, which is a significant health risk in some areas.
Food sources, as certain foods contain trace amounts of radioactive isotopes.
Half-Life
Concept: The half-life of a radioactive material is the time required for half of the unstable nuclei present in a sample to decay into stable nuclei. Understanding half-lives is essential in various fields, including geology, archaeology (for carbon dating), and medicine (for tracing radioactive isotopes).
Uses of Radioactivity
Applications: Radioactivity has numerous applications in various fields:
Medical Field: Sterilization of food and medical equipment through exposure to high levels of gamma radiation to eliminate bacteria and viruses.
Diagnostic Imaging: The use of radioactive tracers in nuclear medicine for imaging and diagnosis.
Industrial Uses: In gauging applications to measure material thickness and density.
Radiation Safety
Detection: Film badges are often worn by individuals who work in environments with ionizing radiation. Exposure to radiation causes the film to darken:
Alpha radiation: Only the top third of the film turns black.
Beta radiation: The top two-thirds of the film darkens.
Gamma radiation: The entire film is exposed dark, indicating penetration through all layers of protective covering.
Minimizing Exposure to Radiation
Safety Measures: It's crucial for those working with ionizing radiation to implement various safety measures:
Utilize lead-lined aprons during X-ray procedures to limit exposure to non-target body parts.
Stand behind lead shielding to absorb radiation during medical procedures or industrial applications.
Maintain a safe distance from radiation sources, as intensity decreases significantly with increased distance.
Reduce time of exposure to radioactive substances whenever possible.
Employ robotic systems in high-radiation areas, such as in nuclear power stations, to minimize human exposure.
Use tongs and other tools for handling radioactive materials to avoid direct contact.
Wear gloves and protective lab coats when there is a risk of contamination, which mitigates the risk of transferring radioactive materials to skin or clothing, despite not fully protecting against beta or gamma radiation.