GLOBAL CHALLENGES (OCR CHEMISTRY)
Chemistry plays a pivotal role in addressing global challenges by optimizing processes and product development. From understanding the intricate balance of nutrients in fertilizer production to mitigating the corrosive effects on metals, chemical principles underpin innovations in various industries. The selection and modification of materials based on their properties further exemplify chemistry's impact on creating sustainable and efficient solutions for a wide range of applications.
1. Improving Processes and Products
Extracting Iron and Copper
Ores
Unreactive metals, such as gold, are found in the Earth’s crust as the uncombined elements. However, most metals are found combined with other elements to form compounds.
An ore is a rock that contains enough of a metal or a metal compound to make extracting the metal worthwhile:
Low grade ores contain a small percentage of the metal or its compound
High grade ores contain a larger percentage
Most metals are extracted from ores found in the Earth’s crust. It is more expensive and wasteful to extract a metal from a low grade ore, but a lot of the high grade ores have already been used.
Extraction Methods
The extraction method used depends upon the metal’s position in the reactivity series. In principle, any metal could be extracted from its compounds using electrolysis. However, large amounts of electrical energy are needed to do this, so electrolysis is expensive.
If a metal is less reactive than carbon, it can be extracted from its compounds by heating with carbon. Copper is an example of this. Copper mostly occurs as sulfide ores, which are heated in air to convert them to copper oxide. Molten copper can be produced from copper oxide by heating with carbon:
copper oxide + carbon → copper + carbon dioxide
2CuO(s) + C(s) → 2Cu(l) + CO2(g)
Copper oxide is reduced as carbon is oxidized, so this is an example of a redox reaction. The impure copper is purified by electrolysis.
The table summarizes the extraction methods used for different metals.
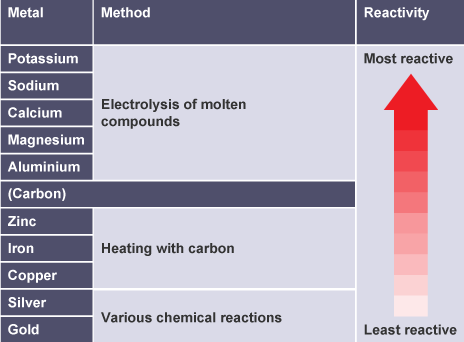
Although an unreactive metal is found as the uncombined element, chemical reactions are often needed to remove other elements that might contaminate it.
Extracting Iron
Iron is extracted from iron ore in a large container called a blast furnace. Iron (III) oxide is reduced to molten iron when it reacts with carbon. For example:
iron(III) oxide + carbon → iron + carbon monoxide
Fe2O3(s) + 3C(s) → 2Fe(l) + 3CO(g)
In the high temperatures of a blast furnace, carbon monoxide also reduces iron(III) oxide:
iron(III) oxide + carbon monoxide → iron + carbon dioxide
Fe2O3(s) + 3CO(s) → 2Fe(l) + 3CO2(g)
This method of extraction works because carbon is more reactive than iron, so it can displace iron from iron compounds.
Extracting a metal by heating it with carbon is cheaper than using electrolysis.
Extracting Aluminum
Aluminum is more reactive than carbon so it must be extracted from its compounds using electrolysis.
Even though aluminum is more abundant than iron in the Earth’s crust, aluminum is more expensive than iron. This is mainly because of the large amounts of electrical energy used in the extraction process.
Electrolysis of Aluminum Oxide
The Electrolyte
Aluminum ore is treated to produce purified aluminum oxide. The electrolytes used in electrolysis are ionic compounds and are either:
in the molten state
dissolved in water
Aluminum oxide is insoluble in water, so it must be molten to act as an electrolyte. However, the melting point of aluminum oxide is high. A lot of energy must be transferred to break its strong ionic bonds, which would be expensive.
To reduce costs, powdered aluminum oxide is dissolved in molten cryolite. This ionic compound melts at a lower temperature than aluminum oxide.
The Electrolysis Process
The diagram shows an electrolysis cell used to extract aluminum. Both electrodes are made of graphite, a form of carbon with a high melting point and which conducts electricity.
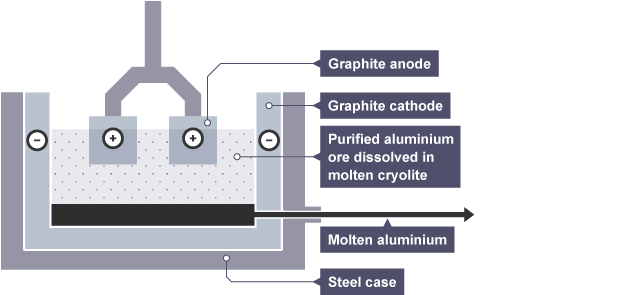
During electrolysis:
at the cathode, aluminum ions gain electrons and form aluminum atoms
at the anode, oxide ions lose electrons and form oxygen gas
The oxygen reacts with the carbon anodes, forming carbon dioxide. So the anodes gradually burn away. They must be replaced frequently, adding to the cost of producing aluminum.
Recycling Materials
Waste materials such as polymers and metals are often disposed of in landfill sites. Waste polymers may be incinerated. However, these disposal methods waste valuable resources, including these raw materials:
crude oil for making most polymers
metal ores for making most metals
These are finite resources - they form extremely slowly or are no longer being made. Recycling reduces the problems of disposal, and also conserves raw materials.
Recycling Polymers and Metals
Recycling involves collecting used items and producing new materials from them. The first steps usually needed are:
collecting used items
transporting the used items to a recycling center
breaking up the items and sorting the different materials
Polymers
Recycling polymers also involves:
sorting the different polymers from one another
melting the waste polymer
forming the polymer into a new product
Metals
Recycling metals also involves:
sorting the different metals from one another
melting the metal and removing impurities from the molten metal
solidifying the metal in ingots
The ingots can then be used to manufacture new metal items.
Life-cycle Assessments
A life-cycle assessment or LCA is a ‘cradle-to-grave’ analysis of the impact of a manufactured product on the environment. There are many detailed stages but the main ones are:
obtaining the raw materials needed
manufacturing the product
using and maintaining the product
disposing of the product at the end of its useful life
Use of water, resources, energy sources, and production of some wastes can be fairly easily quantified.
Allocating numerical values to pollutant effects is less straightforward and requires value judgements, so LCA is not a purely objective process.
Selective or abbreviated LCAs can be devised to evaluate a product but these can be misused (e.g. in support of claims for advertising purposes).
2. Making Fertilizers
Fertilizers
Fertilizers provide mineral ions needed for healthy growth in plants. As plants grow, they absorb mineral ions from the water in the soil through their root hair cells. Over time, the concentration of these ions decreases, so farmers and gardeners add fertilizers to the soil.
Nitrogen, Phosphorus, and Potassium
Fertilizers may contain nitrogen, phosphorus and potassium compounds to promote plant growth. Fertilizers that supply all three elements are often called NPK fertilizers, after the chemical symbols for these three elements.
Fertilizer compounds must be soluble in water so they can be absorbed by the root hair cells:
ammonium ions, NH4+, and nitrate ions, NO3-, are sources of soluble nitrogen
phosphate ions, PO43-, are a source of soluble phosphorus
all common potassium compounds dissolve in water to produce potassium ions, K+
The table shows some examples of fertilizers, their formulae and the essential elements they provide.
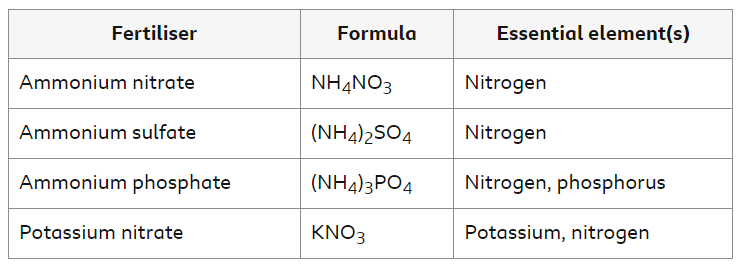
The Haber Process
Making Ammonia
Ammonia is an important industrial product used to make fertilizers, explosives and dyes. It is manufactured using the Haber process. This involves a reversible reaction between nitrogen and hydrogen:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The reaction can reach a dynamic equilibrium.
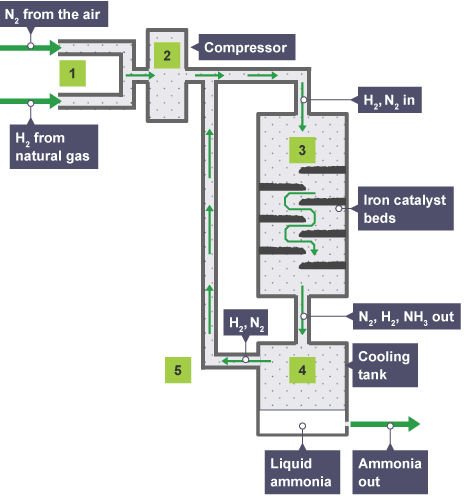
In the Haber Process:
Nitrogen (extracted from the air) and hydrogen (obtained from natural gas) are pumped through pipes.
The pressure of the mixture of gasses is increased to 200 atmospheres.
The pressurized gasses are heated to 450 °C and passed through a tank containing an iron catalyst.
The reaction mixture is cooled so that ammonia liquefies and can be removed.
Unreacted nitrogen and hydrogen are recycled.
Making Fertilizers
Ammonium salts are sources of soluble nitrogen, so they can be used as ‘nitrogenous’ fertilizers. They are manufactured on an industrial scale, but they can also be made in the laboratory on a smaller scale.
Ammonium nitrate, NH4NO3, and ammonium sulfate, (NH4)2SO4, are two nitrogenous fertilizers.
Making Ammonium Sulfate in the Lab
Ammonium sulfate can be made in the lab using dilute ammonia solution and dilute sulfuric acid:
ammonia + sulfuric acid → ammonium sulfate
2NH3(aq) + H2SO4(aq) → (NH4)2SO4(aq)
Both reactants are soluble, so titration must be used.
Here is an outline of one way to make ammonium sulfate in the lab. Eye protection must be worn.
Put some dilute sulfuric acid into a beaker.
Add a few drops of methyl orange indicator.
Add dilute ammonia solution drop by drop, stirring in between.
Continue step 3 until the color permanently changes from red to yellow.
Add a few more drops of dilute ammonia solution.
Pour the reaction mixture into an evaporating basin, and heat carefully over a boiling water bath.
Stop heating before all the water has evaporated and leave aside for crystals to form.
Pour away excess water and leave the crystals to dry in a warm oven, or pat dry with filter paper.
3. Corrosion of Metals
Corrosion
Metals can oxidize in air. They react with oxygen and form metal oxides. For example, sodium is a very reactive metal.
When sodium is cut or scratched, its freshly exposed shiny surface rapidly turns dull as a thin layer of sodium oxide forms:
sodium + oxygen → sodium oxide
4Na(s) + O2(g) → 2Na2O(s)
Other metals may oxidize more slowly. Gold and other very unreactive metals do not oxidize in air at all.
Corrosion happens when a metal continues to oxidize. The metal becomes weaker over time, and eventually all of it may become metal oxide.
Rusting
Rusting is a specific example of corrosion, which occurs when iron or steel reacts with oxygen and water:
iron + oxygen + water → hydrated iron(III) oxide
Hydrated iron(III) oxide is the orange-brown substance seen on the surface of rusty objects.
Preventing Corrosion
Rusting can be prevented by creating a physical barrier to oxygen and water. Ways to do this include:
Painting
oiling and greasing
coating with plastic
Different methods are used depending on the situation.
Electroplating
Electroplating involves using electrolysis to put a thin layer of a metal on the object:
the cathode is the iron or steel object
the anode is the plating metal
the electrolyte contains ions of the plating metal
For example, steel cutlery can be electroplated with silver using a silver anode and silver nitrate solution. Electroplating improves the corrosion resistance of metal objects. It also improves their appearance and may be used to produce gold-plated jewelry.
Galvanizing
When iron is coated in zinc, the process is called galvanizing. The zinc layer stops oxygen and water reaching the iron. Zinc is more reactive than iron, so it also acts as a sacrificial metal. This protection works, even if the zinc layer is scratched.
Alloys
An alloy is a mixture of two or more elements, where at least one element is a metal. Many alloys are mixtures of two or more metals.
Alloy Strength
Converting pure metals into alloys often increases the strength of the product. For example, brass is an alloy of copper and zinc. It is stronger than copper or zinc alone.
Explaining Alloy Strength
Solid metals have a regular lattice structure. When a force is applied to a metal, layers of atoms can move past each other. The more difficult it is for the layers to move, the more force is needed and the stronger the metal.
Copper and zinc atoms are different sizes. This distorts the regular lattice structure in brass, so layers of atoms cannot slide over each other so easily. This makes brass stronger than copper or zinc alone.
Uses of Alloys
Iron is alloyed with other metals to produce a range of alloy steels. Steels have different properties, depending on their composition. For example:
Mild steel is useful for making car body parts because it is easily pressed into shape.
Tool steel is useful for making drill bits because it is hard and not easily damaged by the heating caused by friction during drilling.
4. Materials for Different Uses
Different Materials
Different materials have different properties, but they may also have some properties in common. The table summarizes some of the typical properties of glass and clay ceramics, and metals.
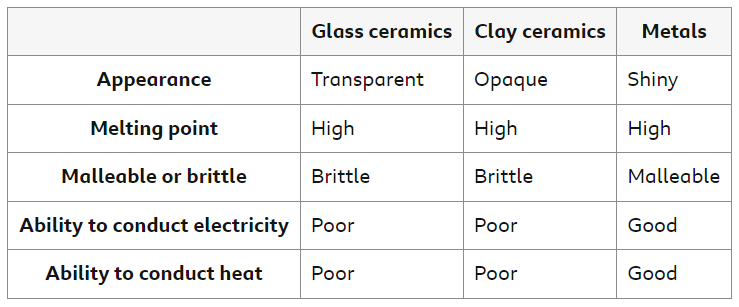
Glass Ceramics
Glass is made by melting sand, then allowing the molten liquid to cool and solidify. Glass is transparent and hard, but it is brittle.
Clay Ceramics
Clay ceramics include brick, china and porcelain. They are made by heating clay to high temperatures, which causes crystals to form and join together. Clay ceramics are often coated with a glaze, which hardens on heating to form a hard, smooth, opaque, and waterproof layer.
Metals
Metals are malleable and ductile, so they can be bent into shape or made into wires without shattering. Unlike glass and clay ceramics, metals are good electrical conductors.
Uses of Polymers
Polymers are poor conductors of electricity and heat, but their other properties vary depending upon the particular polymer. For example, they can be transparent or opaque. They are often tough and flexible, but some are hard and brittle.
Different polymers have different properties. This means that different polymers have different uses.
The table gives examples of polymers, their common names, and their typical properties and uses.
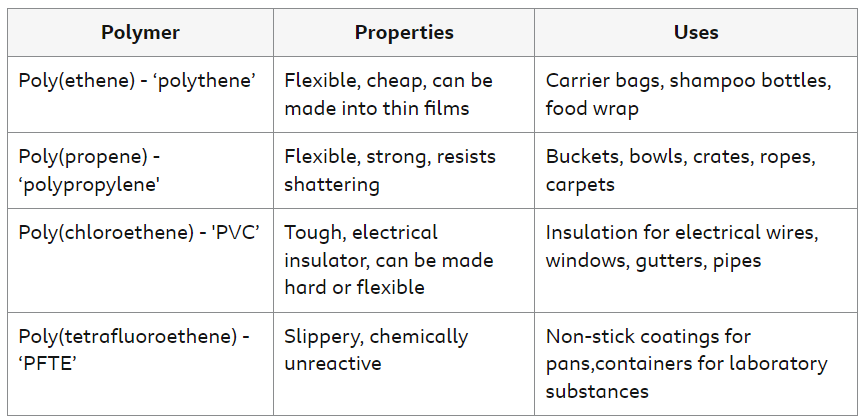
Composite Materials
A composite material consists of two or more materials with contrasting properties. They are combined to produce a material with improved properties.
Most composite materials have two components:
the reinforcement
the matrix, which binds the reinforcement together
The table shows some examples of composite materials.
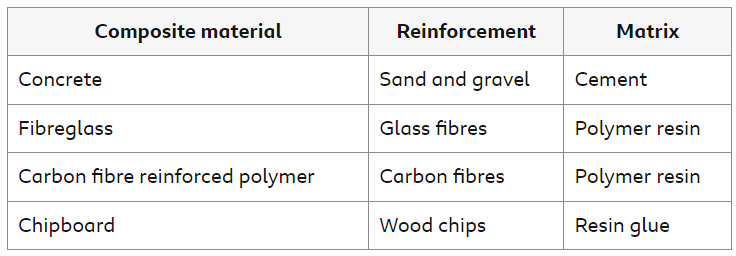
It is often possible to separate the reinforcement from the matrix by physical processes. For example, concrete can be broken up using machinery. This is one stage in recycling the components of concrete.
Fiberglass and Carbon Fiber Reinforced Polymer
The fibers in these composite materials have a low density. They are strong in tension, so they are not easily stretched, but they are brittle.
The polymer resin which binds the fibers together is not strong, but it is stiff and hard wearing. The composite materials show a combination of these properties. They are strong, stiff, hard and lightweight.
Chipboard
Wood itself is a natural composite material. It consists of a reinforcement of cellulose fibers bonded together by a matrix of lignin. The fibers are aligned alongside each other, so wood is stronger in one direction than it is in the other.
Chipboard contains randomly arranged wood chips bonded together by a glue, so it is strong in all directions.
Reinforced Concrete
The properties of concrete can be improved by reinforcing it with steel rods or mesh. The compressive strength of concrete is higher than its tensile strength, but the tensile strength of steel is higher than its compressive strength. The combination of the two materials is strong in tension and in compression. Reinforced concrete is therefore strong and slightly flexible, whereas concrete alone is brittle.
Choosing Materials
The properties of a material determine whether it is suitable for a given use. For any given use, certain properties will be required. There may be more than one suitable material to choose from, and the advantages and disadvantages of each one must be evaluated.
This table shows some properties of samples of aluminum alloy, mild steel and timber:
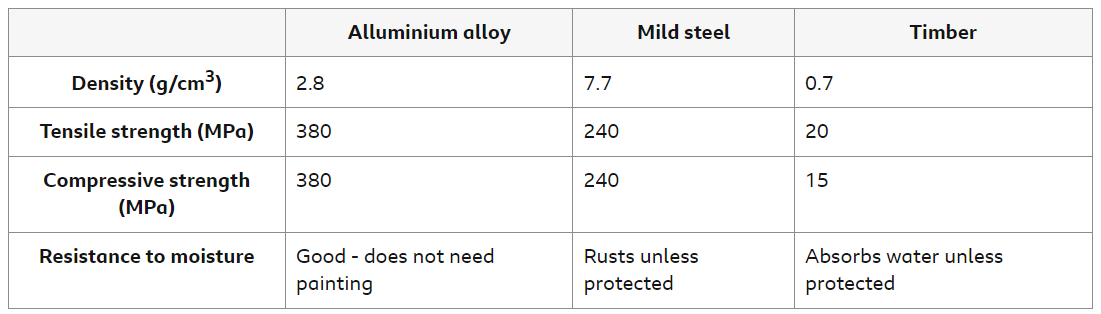
The mild steel is the weakest metal, although timber is the weakest material of the three. Mild steel has a lower tensile strength than aluminum, so it stretches more easily when forces are applied. It has a lower compressive strength, so it is crushed more easily when forces are applied.
Timber has the lowest density, so a wooden frame could be the most lightweight. However, timber is the weakest material, so the frame will need to be thicker or use more pieces. This might make the wooden frame heavier than a metal frame.
Mild steel is almost as strong as aluminum alloy. However, it is 2.75 times denser than aluminum, so a steel frame is likely to be much heavier. A heavier carriage may reduce the number of carriages the train can pull, or reduce its acceleration so that journey times increase.
The aluminum alloy is four times denser than timber, but at least 19 times stronger. An aluminum frame is likely to be the most lightweight of the three materials. Aluminum alloy has a good resistance to moisture. Unlike mild steel and timber, it does not need protection. Overall, aluminum alloy would be the best choice.