3. Macromolecules in solution
Macromolecules- Large molecules built up by small molecules (monomers), that are covalently linked
Examples:
Biological polymers:
- Polysaccharides such as starch, cellulose etc.
- Sugar monomers
- Lignin
- Phenolic monomers
Proteins: Not strictly polymers
- Amino acid monomers
Synthetic polymers:
- Polyethylene (PE)
- Polyethylene glycol (PEG)
- Polyvinylalcohol (PVA)
- Polystyrene (PS)
- Polyacrylamide etc.
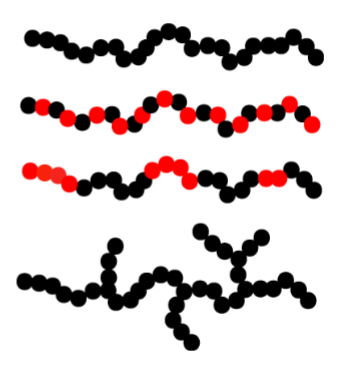
Homopolymer- same type of monomer
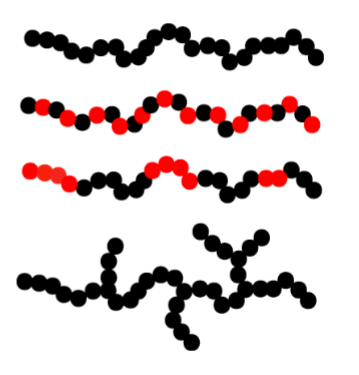
Copolymer- alternating monomer
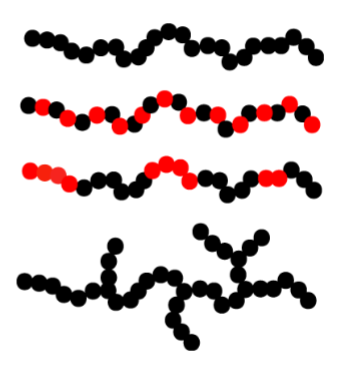
Block copolymer- alternating in block→ making them surface-active
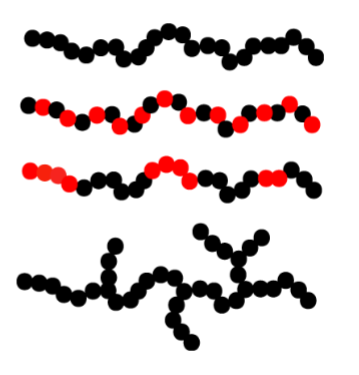
Branched homopolymer- branching can occur for others
Average molecular weight
• Molecular weight (molar mass) is given by:
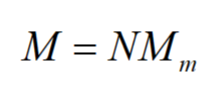
• Biopolymers and most synthetic polymers are polydisperse (different chain length).
– Average molar mass (molecular weight).
– Average molar mass depends on the method used to determine it.
Polymer size - Hydrodynamic radius
Hydrodynamic radius is obtained from the Stokes-Einstein equation. It describes how diffusion of a particle or polymeric structure depends on its radius.
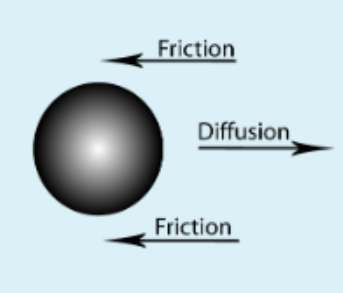
Stokes-Einstein
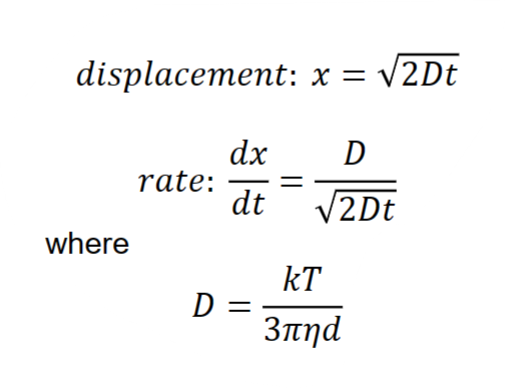
D= diffusion coefficient [m2/s]
k= Boltzmann constant (J/K)=1.38×10−23 J/K
T= temperature (K)
η=dynamic viscosity of continuous phase [Pa•s]
d=hydrodynamic diameter of particle [m]
Polymer size - Radius of gyration
Based on the distance of each monomer unit to the polymer’s center of mass. Used for random coil polymer
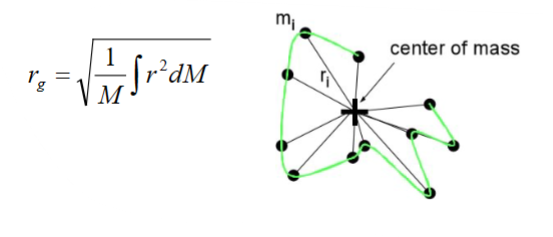
Debey’s definition of radius of gyration- It is most common one. Distance of every mass to the center of mass. The average distance is rg
rg=average distance
Mi=molar mass
ri=distance to the center of mass
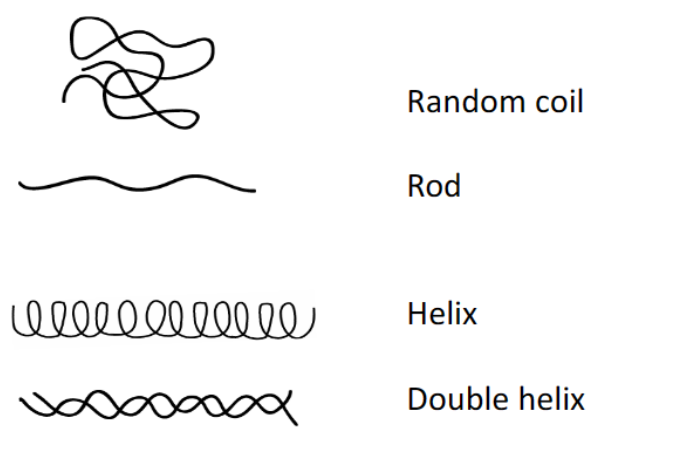
Polymer conformation in dilute solutions
Conformation in solutions depends on:
- Interactions with solvent molecules
- pH
- Salt
- Temperature
- Interactions with other polymer molecules
Really likes solvent: polymer swell
Doesn’t like it as much: polymer minimise
Conformation in dilute solutions
Rod: stiff
Double helix: two molecules
Flory-Huggins theory- Describes solubility of a polymer and thermodynamics of macromolecular solutions.
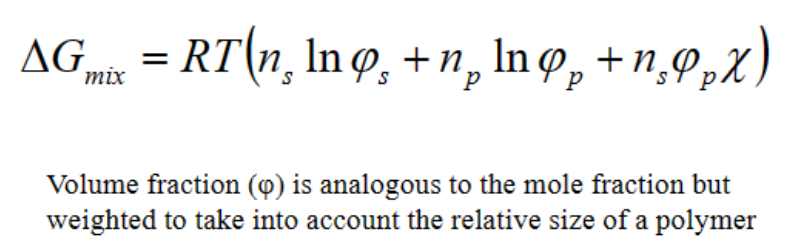
Volume fraction (φ) is analogous to the mole fraction but weighted to take into account the relative size of a polymer
The last sum: interaction between polymer and solvent
• The Flory-Huggins parameter (χ)
– 0.1< χ<0.5 ”Good” solvent (molecule swell)
– χ ≈ 0.5 ”θ-solvent” (transition between good and poor solvents) (reduce it bellow to precipitate, increase to allow it dissolve)
– χ > 0.5 ”Poor” solvent (molecule shrink)
– χ >> 0.5 Insoluble
• χ can change with temperature, pH, ionic strength etc.
Phase behaviour of macromolecule solutions
Polymer 1 and polymer 2 are chemically different
Miscibility: polymers like the solvent, don’t care about each other
Segregative phase separation: Polymers don’t like each other but like solvent
- The rule for mixtures of non-ionic polymers
Associated phase separation: Polymers like each other better than solvents (e.g polymer could have opposite charges)
- Polyelectrolytes of opposite charge, proteins-polysaccharides
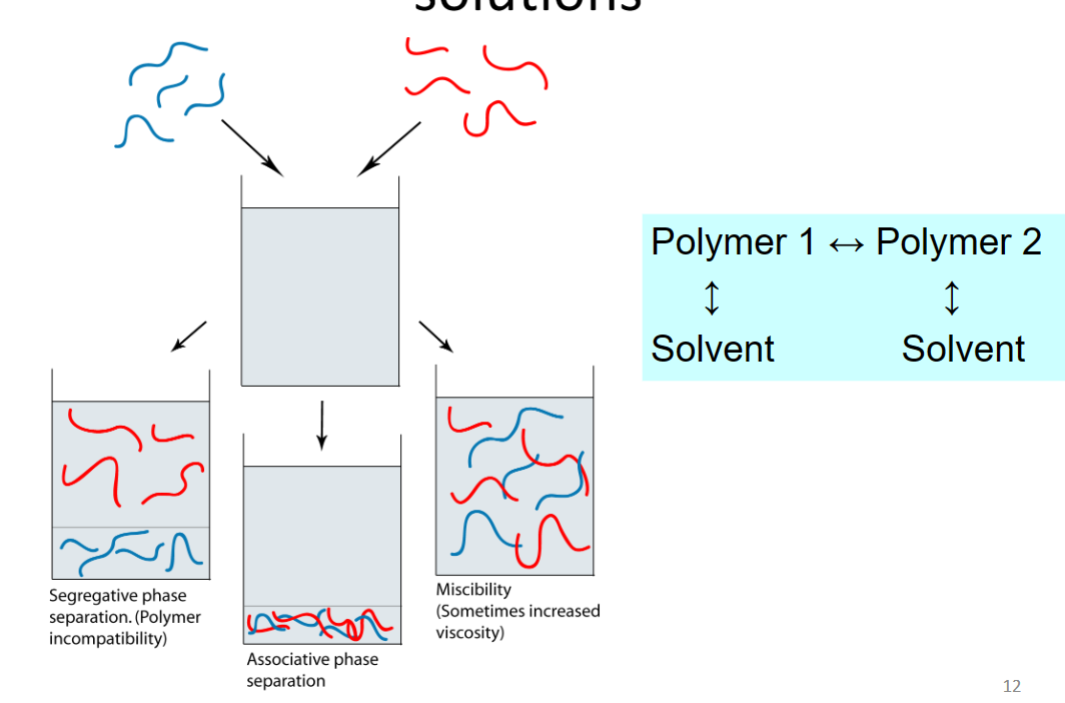
Phase behaviour of macromolecule solutions
• Cooling and heating can cause phase separation.
• θ-temperature.
• Example of phase diagram - Polyethylenoxide
Horizontal axis is concentration
The highest molecular weight is most sensitive high temperature
Interactions between water molecules decreases at that temperature, molecules becomes soluble again
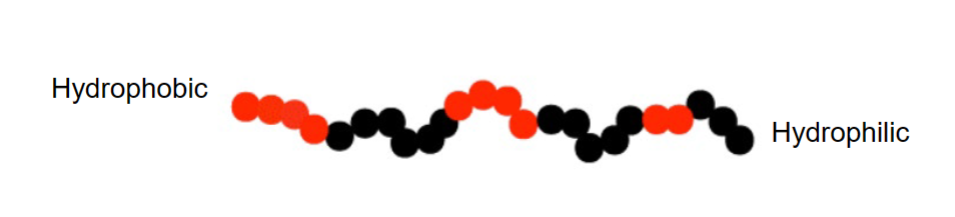
Surface active macromolecules
- Proteins and polymers can be surface active
- Hydrophobic domains are excluded from a polar phase
- Hydrophilic domains ensure the solubility of the molecule
- May associate and form aggregates in the solution
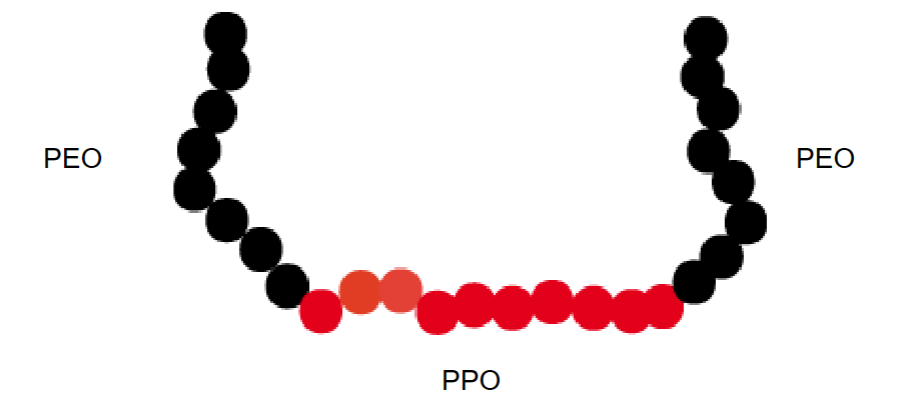
Example: Block co-polymers (Pluronic).
PEO=hydrophilic
PPO=hydrophobic
Applications of macromolecules
• Thickening to increasing viscosity to preferred value and flow properties in liquid formulations. Example: Pharmaceutics, Foods and Paints and coatings
• Stabilization of dispersions
Rheology - Rheology is the study of the flow and deformation of matter, including liquids, solids, and soft materials, under applied forces or stresses.
Shear stress- Shear stress is the external force applied parallel to the surface, which causes deformation.
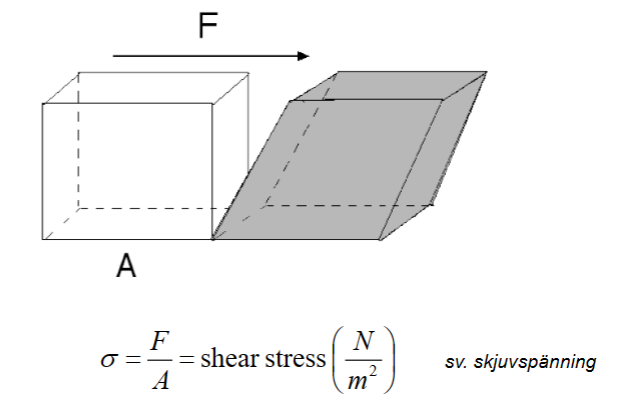
σ=shear stress [Pa]
F=applied external force [N]
A=cross-sectional area [m2]
Shear rate- Shear rate 𝛾 is the rate at which strain changes over time, which is the rate of deformation.
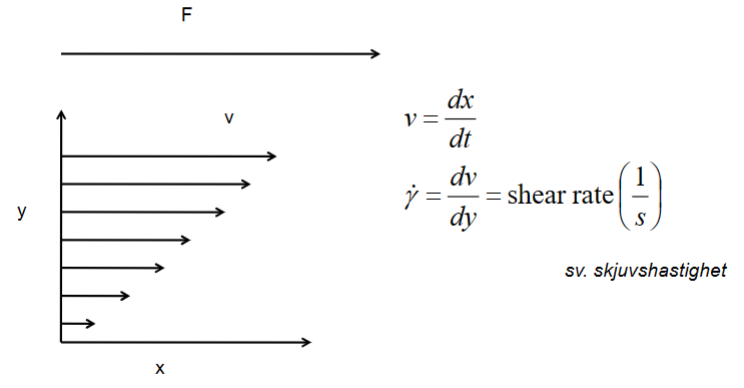
γ=shear rate [1/s]
v=velocity [m/s]
y=distance between moving plates [m]
Viscosity
In a pure liquid: friction between molecules
In a solution of polymers: resistance to a deforming flow compared to pure solvent → increase in viscosity
Normally for polymer: Shear-thinning behaviour arises from orientation of polymer chains
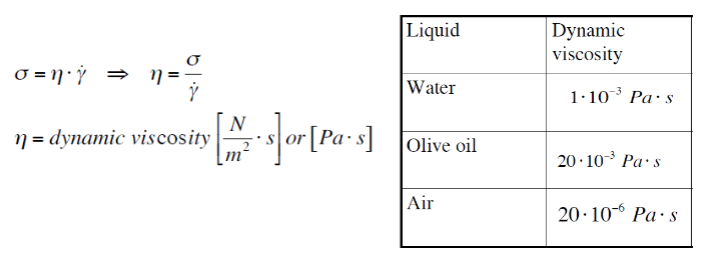
Dynamic viscosity-
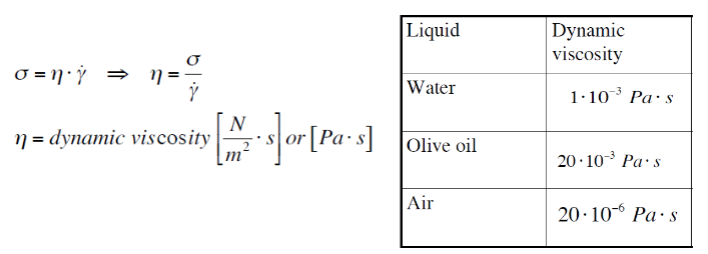
γ=shear rate [1/s]
σ=shear stress [Pa]
η=dynamic viscosity [Pa・s=N・s/m2]
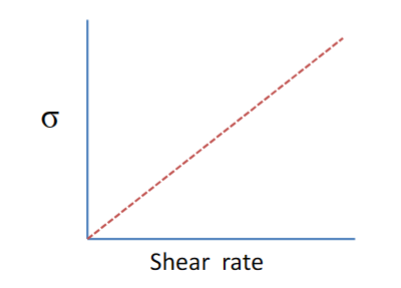
Newtonian flow
• Shear stress proportional to shear rate → viscosity independent of shear rate.
• Low molar mass liquids (water, organic solvent etc.).
Viscosity increase in dispersions arises from disturbances in the flow field
Orientation of particles in the flow
What happens to the viscosity? The particles align themselves with the flow
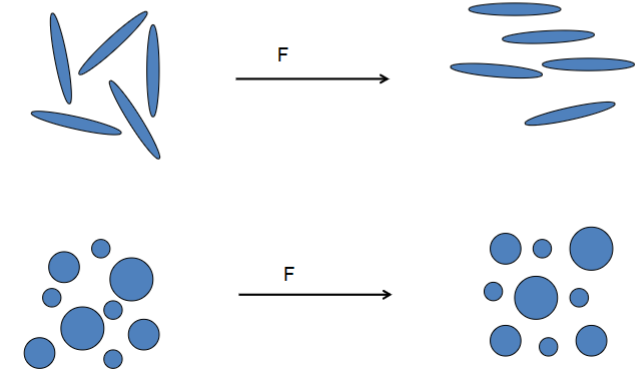
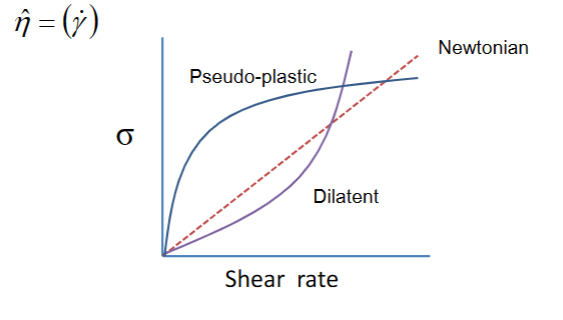
Non-Newtonian flow
• The viscosity is shear rate dependant.
• Pseudo-plastic flow: The viscosity decreases with increasing shear rate (as known as shear-thinning). For example paint
• Dilatent flow: The viscosity increases with increasing shear rate. For example wet sand (particle volume fraction increase locally)
• Apparent viscosity
Non-newtonian behaviour arises from disturbances in the flow pattern caused by sample components
• The power law can be used to describe non-newtonian flow
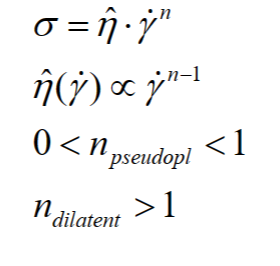
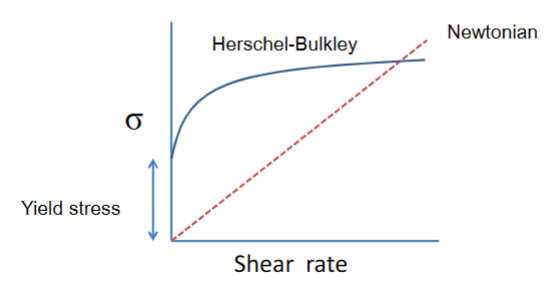
Yield stress
• Above the yield stress the system starts to flow.
• Below the yield stress the system appears solid (i.e. gel)
Herschel-Bulkley: Tooth paste, tomate ketchup
Viscosity of macromolecule solutions
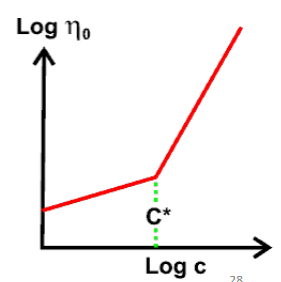
• The overlap concentration (c*) is the point where solution viscosity increases sharply
with polymer concentration
• c* depends on the volume occupied by polymer in the solution.
– Large volume → lower c*
– Small volume → higher c*
• Note that the volume is 3D!
– i.e. rod-like conformation occupies a large volume
Viscosity of macromolecule solutions
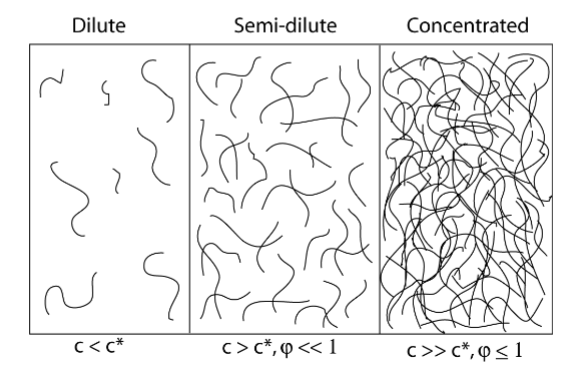
• Concentration regimes
Thixotropy- Thixotropic fluids are a type of non-Newtonian liquids that exhibit shear thinning during increased shear rate and a slow recovery to the original viscosity after the shear rate is removed.
• Time-dependent viscosity decrease during constant shear
• Obtained with associative thickeners – dissociation and orientation give a decrease in viscosity
• The viscosity is recovered when shearing/deformation stops
• Example: Important for controlling paint viscosity and application
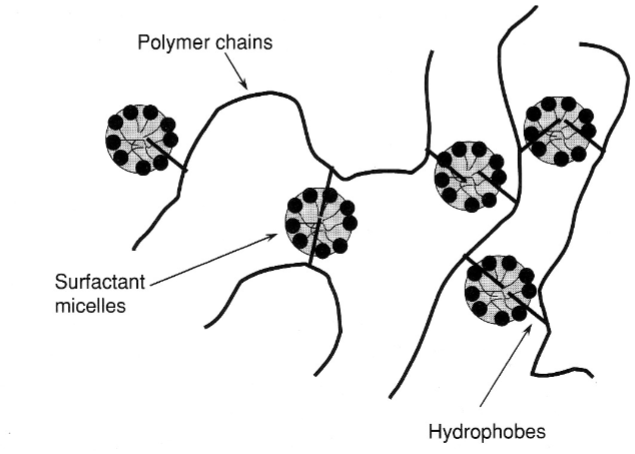
Associative thickeners
Hydrophobic interaction can play a role in polymer network formation
Surfactant micelles can associate with hydrophobic groups