Topic 4 Regents Chemistry Review: Physical Behavior of Matter
Topic Overview
In this chapter you will first examine the solid, liquid, and gaseous phases of matter. Next, you will study how to calculate the heat exchanged during heating, cooling, and phase changes. The Kinetic Molecular Theory will then be presented to explain the behaviors of gases. Finally, you’ll learn about the various means of separating mixtures.
Phases of Matter
An element, compound or mixture may exist in the form of a solid, liquid, or a gas. These three forms are called the phases of matter. The solid phase contains matter that is held in a rigid form. Because of this rigid form, a substance in the solid phase has a definite volume and shape. Strong attractive forces among the particles in a solid hold the particles in fixed locations. True solids have a crystalline structure.
Particles in the liquid phase are not held together as rigidly as those in the solid phase. Liquid phase particles are able to move past one another. The mobility of the particles prevents liquids from having a definite shape. The particles, however, are held together with sufficient attractive force to give a liquid a definite volume.
Particles in the gaseous phase have minimal attractive forces holding them together. Due to this lack of attraction among particles, gases have neither a definite shape nor a definite volume. Gases spread out indefinitely unless they are confined in a container. In a closed container, the gaseous particles always expand to fill the volume of the container. A vapor is the gaseous phase of a substance that is a liquid or a solid at normal conditions.
Heating and Cooling Curves
At minute 0 shown on Point A on the graph, the temperature of the solid is 10°C. Heat is then added to the substance at a constant rate. From minute 0 to minute 2, the temperature rises at a constant rate until the temperature of the solid reaches its melting point (B). During this portion of the process (AB), the kinetic energy of the substance is increasing.
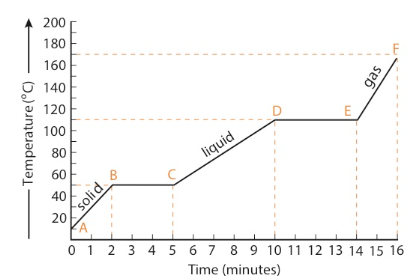
Eventually some of the particles in the substance possess enough kinetic energy to break the bonds holding them in the solid phase; melting, also known as fusion, begins at Point B. During the melting process (BC), the temperature remains constant even though heat is still being added at a constant rate. During this time, the heat absorbed by the substance in the form of potential energy. Both solid and liquid phases of the substance are present during the melting process. As time goes on, the amount of liquid continually increases and the amount of solid continually decreases. Because the liquid phase of a substance has more potential energy than the solid phase, the potential energy of the substance increases during the process. The amount of heat needed to convert a solid at its melting point to a liquid is called the heat of fusion.
When all of the solid has melted (C) and only the liquid phase is present, the temperature once again begins to rise. This temperature rise is due to the increase in kinetic energy of the substance. The temperature continues to rise until the boiling point is reached (D). Boiling, also known as vaporization, begins as some of the particles in the liquid have enough kinetic energy to break free from the attractive forces holding them in the liquid phase. These particles escape the liquid and enter the gas phase. Once boiling begins, the temperature remains constant as the substance's potential energy increases. During this phase change, both the liquid and gaseous (vapor) phases are present. Because the gaseous phase of a substance has more potential energy than the liquid phase, the potential energy of the substance increases as heat is absorbed during the boiling process. If heat is added to the substance in its gas phase, the temperature of the gas begins to rise (EF).
Heating Curve (Endothermic) Summary
AB: heating of a solid, one phase present, kinetic energy increases BC: melting of a solid, two phases present, potential energy increases, kinetic energy remains constant
CD: heating of a liquid, one phase present, kinetic energy increases
DE: boiling of a liquid, two phases present, potential energy increases,
kinetic energy remains constant
EF: heating of a gas, one phase present, kinetic energy increases
If a gas at high temperature is allowed to cool at a constant rate, a cooling curve results. Note that the reverse of boiling is called condensation, and the reverse of melting is called freezing. Freezing is also called solidification.
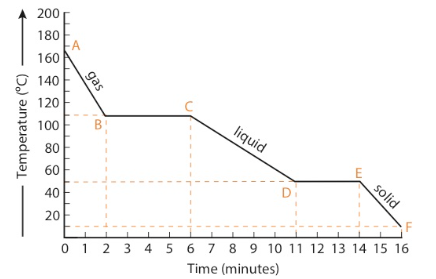
Cooling Curve (Exothermic) Summary
AB: cooling of a gas (vapor), one phase present, kinetic energy decreases
BC: condensation of the gas (vapor) to liquid, two phases present, potential energy decreases, kinetic energy remains constant
CD: cooling of a liquid, one phase present, kinetic energy decreases
DE: solidification (freezing) of a liquid, two phases present, potential energy decreases, kinetic energy remains the same
EF: cooling of a solid, one phase present, kinetic energy decreases
Sublimation and Deposition
Heating and cooling curves show the normal transitions between phases. Some substances, however, change directly from a solid to a gas without passing through a noticeable liquid phase. An example is solid carbon dioxide (CO2), which changes from a solid to a gas at normal atmospheric pressure. This process, in which a solid changes directly into a gas, is called sublimation. A substance that undergoes sublimation is said to sublime. The reverse of sublimation, in which a gas changes directly into a solid is called deposition.
Temperature Scales
The temperature of a substance is a measure of the average kinetic energy of its particles. The particles of all substances at the same temperature have the same average kinetic energy. The temperature difference between two bodies indicates the direction of heat flow. That is, whenever two objects with different temperatures are in contact, heat flows from the object at the higher temperature to the object at the lower temperature. The heat flow continues until the two objects are at the same temperature.
The average kinetic energy depends only on the temperature of the substance, and not on the nature or amount of the material. Thus, 10 g of H2O at 50°C has greater average kinetic energy than 500 g of Fe(s) at 20°C.
Temperature is measured using a thermometer. Thermometers are calibrated by establishing two fixed reference points; the distance between them is then divided into the desired number of units. The fixed points on common thermometers are the freezing and boiling points of water. The freezing point of water is the ice-water (solid-liquid) equilibrium temperature at normal atmospheric pressure (101.3 kPa). The boiling point of water is the water-steam (liquid-gas) equilibrium temperature at normal atmospheric pressure.
Because there are an equal number of divisions between the fixed reference points of both Celsius and Kelvin scales, a change of one degree Celsius is equal to a change of one Kelvin. The Celsius and Kelvin Scales are related by the following equation
K = °C + 273
Although often confused, heat and temperature are not the same. Heat is a measure of the amount of energy transferred from one substance to another. Heat is measured in units of calories or joules. Temperature is a measure of the average kinetic energy of a substance's particles, and is measured in degrees Celsius or in Kelvins. An example of the difference between heat and temperature involves the melting of ice. It requires more energy to melt 10 g of ice than it does to melt 1 g of ice, yet in both cases the temperature of the ice does not change.
Measurement of Heat Energy
The amount of heat given off or absorbed in a reaction can be calculated using the following equation:
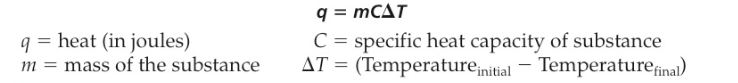
Heat Of Fusion
The amount of heat needed to convert a unit mass of a substance from solid to liquid at its melting point is called the heat of fusion. The heat of fusion of solid water (ice) at 0°C and 1 atmosphere is 334 J/g. The heat absorbed by the substance during the melting process increases the potential energy of the substance without increasing the average kinetic energy of the substance's particles. Because there is no change in kinetic energy, there is no temperature change during the process.
Heat Of Vaporization
During the boiling process, a substance in the liquid phase is converted to the gaseous (vapor) phase. The temperature remains constant during the boiling process even though energy is constantly added. The heat energy increases the potential energy of the particles in the gaseous phase. The amount of heat needed to convert a unit mass of a substance from its liquid phase to its vapor phase at constant temperature is called its heat of vaporization.
As heat is added, the particles absorb sufficient energy to overcome the attractive forces holding them in the liquid phase. The potential energy of the system increases as the temperature remains constant. The heat of vaporization of water at 100°C and 1 atmosphere is 2260 J/g. The condensation process is the reverse of boiling process. Therefore the heat of condensation is also 2260 J/g. Condensation is an exothermic process.
Behavior of Gases
Scientists construct models to explain the behavior of substances. While the gas laws describe how gases behave, they do not explain why gases behave the way they do. The kinetic molecular theory (KMT) is a model or theory that is used to explain the behavior of gases. This theory describes the relationships among pressure, volume, temperature, velocity, frequency, and force of collisions.
Kinetic Molecular Theory
The major ideas of kinetic molecular theory are summarized in the following statements:
Gases contain particles (usually molecules or atoms) that are in constant, random, straight-line motion.
Gas particles collide with each other and with the walls of the container. These collisions may result in a transfer of energy among the particles, but there is no net loss of energy as the result of these collisions are said to be perfectly elastic.
Gas particles are separated by relatively great distances. Because of this, the volume occupied by the particles themselves is negligible and do not need to be accounted for.
Gas particles do not attract each other.
Relationship of Pressure and Numbers of Gas Particles The kinetic molecular theory easily explains why gases exert pressure. Not only do gas molecules collide with each other, but they also collide with the walls of their container. These collisions with the container wall exert a force over the surface area of the wall-the particles exert pressure on the wall. For example, if you add more air to a bicycle tire, the pressure is increased. The greater the number of air particles, the greater the pressure. Pressure and the number of gas molecules are directly proportional.
Relationship of Pressure and Volume of a Gas Picture a cylinder with a piston at one end. If the piston can be pushed in, the volume will decrease. The molecules of the gas become more concentrated and hit the walls of the container more often. The pressure increases. If the piston is moved outward so as to increase the volume, the molecules hit the walls less often, causing a decrease in pressure. Thus volume and pressure are indirectly, or inversely, related. If one of the variables (volume or pressure) increases, the other must decrease.
Relationship of Temperature and Pressure of a Gas You may recall that the temperature of a substance is defined as a measure of the average kinetic energy of its particles. As the temperature rises, the kinetic energy increases. This increase is due not to an increase in the mass of the particles, but rather to an increase in their velocity. As the temperature rises, the velocity of the particles increases, causing them to hit the walls of the container more often and with greater force. Thus an increase in temperature causes the pressure to increase. Pressure and temperature are directly related.
Relationship of Temperature and Volume of a Gas If the volume of a container could change while the pressure remained constant, how would volume and temperature be related? As the temperature increases, the molecules push harder on the piston of the container. When the internal pressure of the container exceeds the pressure pushing from the outside, the piston is pushed upward and the volume increases. The piston continues to move until the internal and external pressures are equal. Thus volume and temperature are directly related.
Relationship of Temperature and Velocity You know that as the temperature of a substance increases, its kinetic energy increases. What is the cause of this increase in temperature? Obviously, the masses of the particles do not increase; therefore, it must be the velocity of the particles that increases. The higher the temperature, the greater the average velocity of the particles.
Combined Gas Law Equation The relationships among pressure, temperature, and volume can be mathematically represented by an equation known as the combined gas law.
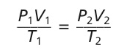
Ideal Gases vs Real Gases
Kinetic molecular theory explains the behavior of gases by using a model gas called an "ideal" gas. When the gas laws are used to solve problems involving "real" gases, the answers obtained often do not exactly match the results obtained in the lab. This is because the ideal gas model does not exactly match the behavior of real gases. These discrepancies arise from the fact that two of the assumptions made by kinetic molecular theory are not exactly correct.
Gas particles do not attract one another. In most cases, the attractive forces between gas particles are so small that they can be disregarded. However, when conditions become extreme, these small forces become important. For example, water molecules in the atmosphere attract each other when temperatures become cold enough. The water molecules combine to form snow or rain.
Gas particles do not occupy volume. Although gas particles themselves occupy a small volume of space under normal conditions, as pressure increases the volume occupied by the particles can no longer be ignored. At high pressures, the increased concentration of particles leads to more frequent collisions and far greater chances of combining.
Separation of Mixtures
The properties of a mixture's components often provide a means by which they can be separated. Density, molecular polarity, freezing point, and boiling point are a few of the properties that can be used to separate the components of mixtures. In this section you will learn some techniques that can be used to separate the components of mixtures.
Filtration
Many mixtures are made up of solids in a liquid. The solids are not dissolved in the liquid, but may be suspended. When allowed to stand undisturbed, the solids will settle to the bottom of the liquid. In some cases, you can separate the two components of the mixture by carefully pouring off the liquid without disturbing the solid. This method, though inefficient, can sometimes be used.
A filter is a material that allows small particles to pass through while trapping larger particles on or in the filter material. In essence, the filter is a material containing holes. Particles that are smaller than the holes pass through, while larger particles cannot pass through the holes and are trapped.
A mixture of a solid in a liquid can often be separated by filtration. As the mixture passes through the filter, the solid is retained on the filter paper, while the liquid passes through. The substance that passes through the filter is called the filtrate, while the substance remaining on the filter is called the residue. Filters are also commonly used to separate mixtures of solids and gases. Air conditioners have filters that allow the air to pass through while trapping solids such as lint and dust. Cars and trucks have similar filters.
Distillation
When solids are dissolved in liquids, making a homogeneous solution, they may be separated by distillation. Figure 4-10 shows a typical distillation apparatus. In the case of a salt and water mixture, the solution is heated and the water begins to boil. The steam passes from the distilling flask into the condensing tube. A water jacket lowers the temperature of the steam. The steam condenses and is collected. The salt remains in the distillation flask.
Chromatography
The process known as chromatography car also be used to separate the components of a mixture. The different components of a mixture often have different attractions for substances not in the mixture. For example when a piece of paper is dipped into some inks, the water in the ink begins to rise by capillary action. The other components of the ink are drawn up along with the water, but they move up the paper at different rates. Because the components of the ink move at different rates, they begin to separate from each other as they move up the paper.
Vocabulary
Condensation - The process by which a gas or vapor changes into a liquid.
Deposition - The direct transition of a substance from a gas to a solid without passing through the liquid phase.
Freezing - The process by which a liquid changes into a solid as a result of losing heat.
Fusion - The process of turning a solid into a liquid, usually by adding heat.
Gaseous Phase - The state of matter in which a substance has no definite shape or volume and fills the entire container it occupies.
Heat - The transfer of energy from one system to another due to temperature differences.
Heat of Fusion - The amount of heat required to change a unit mass of a substance from solid to liquid at its melting point.
Solid Phase - The state of matter characterized by a definite shape and volume, with particles arranged in a regular, repeating pattern.
Sublimation - The process by which a substance transitions directly from a solid to a gas without passing through the liquid phase.
Heat of Vaporization - The amount of heat required to change a unit mass of a substance from liquid to gas at its boiling point.
Temperature - A measure of the average kinetic energy of the particles in a substance.
Kinetic Molecular Theory - A theory that explains the behavior of gases by considering the motion of their particles.
Vaporization - The process by which a liquid changes into a gas, usually by adding heat.
Liquid Phase - The state of matter in which a substance has a definite volume but no definite shape, taking the shape of its container.