lecture 14:MacAB-TolC
MacB was originally found as during screens of E . coli genomes, knocking out MacA and MacB genes made the bacteria sensitive to macrolide which is an antibiotic. It was then known that these genes are responsible for resistance but the mechanism was unknown. It is now known that it forms a tripartite pump. It is now known since AcrAB-TolC has been well studied, these genes can also provide multi drug resistance to drugs such as colistin and kanamycin. It is 32 x more sensitive to erythromycin without this gene and 16x more sensitive to bacitracin. There is consistency with structure to these antibiotics as these have large loop structures and small protein like antibiotics.
The structure was solved by X-ray crystallography and was purified in crystallography. overall, it is a dimer with 4 TM helices, a nucleotide binding domain on the cytoplasmic side - gets ATP bound which gets sandwiched between two nucleotide binding domains (NBD). This transporter doesn't look like any other ABC transporter
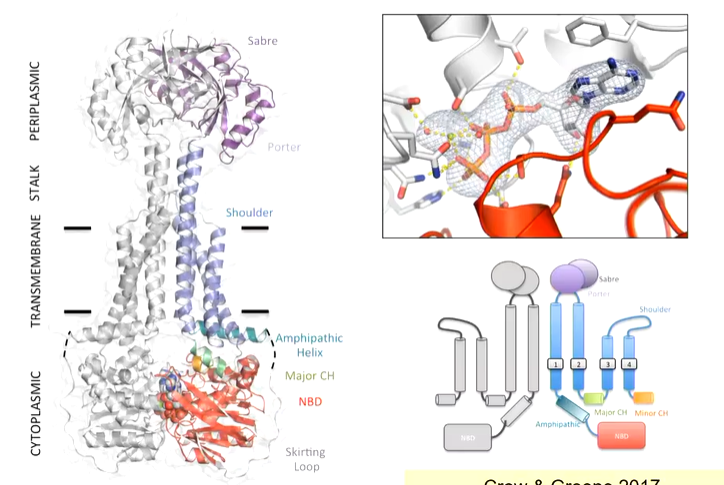
Type 1 and 2 ABC transporters are importers and take substrates from the periplasmic side of the membrane and bring them inside to the cytoplasmic side. There are two different types and although they have the same number of TM helices, the structure is different. Type 3 transporters are toppling transporters and type 4 are the most common while type 5 is less common but are both exporters. There is also a type 6. MacB has a large head group on the periplasmic side and fewer TM helices making its structure different
If you deleted MacB, bacteria became susceptible to erythromycin which works by blocking ribosomes. The simple idea is that MacB must pick it up the erythromycin and scoop it out into the periplasm and into TolC. This isn’t true and instead doesn’t transport any substrates across membranes
Molecular curves of all the other ABC transporters, you see that cavity space changes inside the active site of the transporter.
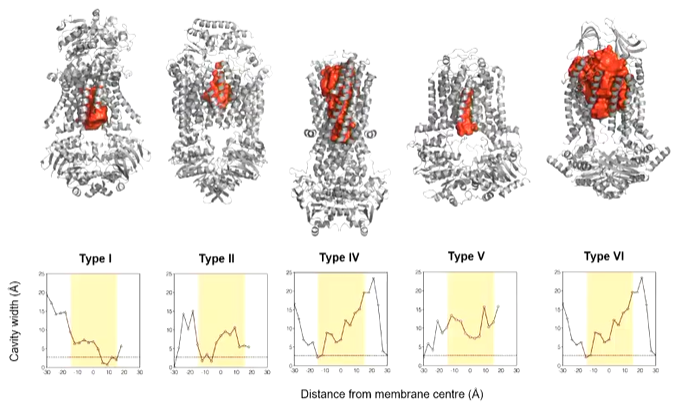
Type 1 and 2 are inward open as they are importers. MacB has a cavity that is too small for erythromycin and is closed on both sides so transport doesn’t happen through here. This suggests that it either doesn’t transport through the membrane or needs large conformational changes.
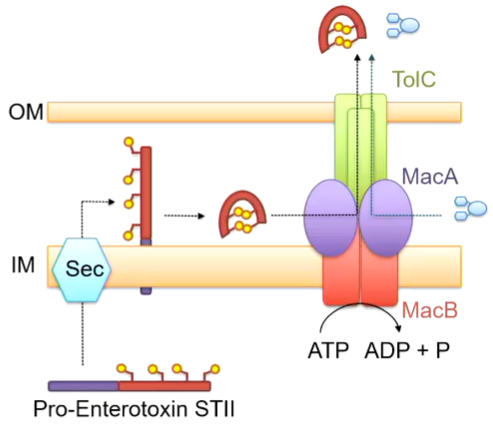
Enterotoxin STII is produced by e. coli that affects baby pigs by binding receptors in the gut to release water for dehydration. This is a small protein toxin that has 2 alpha helices with disulphide bonds. This is made in the cytoplasm but the gene encodes a larger peptide and has a pro sequence at the front that can be recognised as a Sec translocation sequence - directed to the periplasm. It is known that the mature peptide gets rid of the pro sequence so it enters the periplasm and is cleaved here. The disulphide bonds in put in place by another periplasmic enzyme. This suggests that the mature proteins MUST enter the periplasm. It is known that the proteins responsible for this are MacA and MacB as otherwise, it accumulates in the cytoplasm. This suggests that MAcAB-TolC is moving it to the periplasm. Given that this is the case, maybe MacAB-TolC also moves antibiotics from the periplasmic space:
The first thing to find is a binding side on the periplasmic side. A monomer of MacB was looked at and mutations were done at the top of the head domain to see if these had an effect on the ability of e coli to defend against erythromycin. WT e coli can tolerate up to 32 mg/ml of the antibiotic and without MacB only 1 mg/ml is tolerated. Some mutations have little effect on this but some have a large effect. Mapping these balc to the structure, you see that the large impact mutations converge at the same spot which is potentially the part where erythromycin binds. Mutation to the ATP binding site also has a large effect as expected.
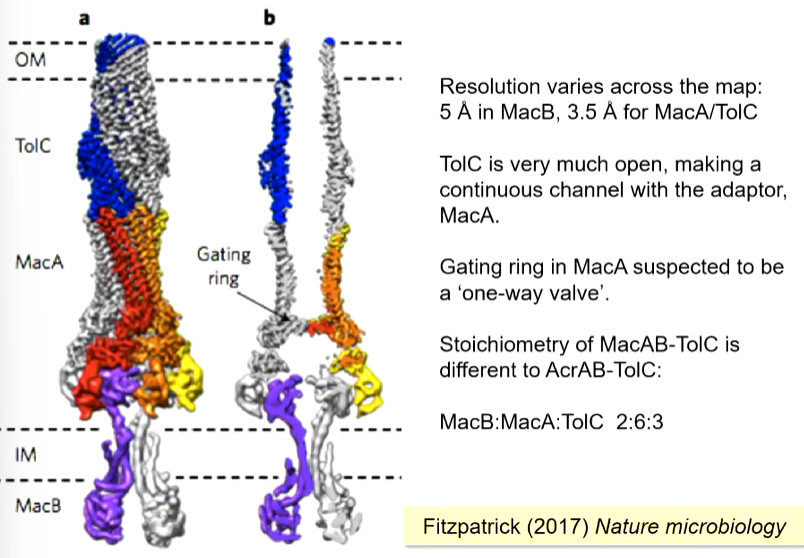
The overall layout is similar to AcrA-TolC but with a MacB instead. If you cut down through the middle, a long channel is seen until you get to the membrane. There is also a gating ring which prevents molecules entering from the outside.
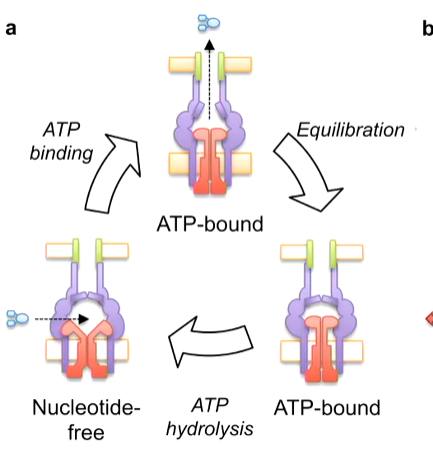
Comparing the ATP bound structure and the unbound structure, there is a conformational change where where the TM region is completely shifted. This was termed mechanotransmission as the conformational change is suspected to drive work on the cytoplasmic side using conformational changes of the dimerisation of the cytoplasmic domain as they bind and hydrolyse ATP. The energy for conformational changes comes from this. There is no way to cross the membrane in both the ATP bound and unbound changes.
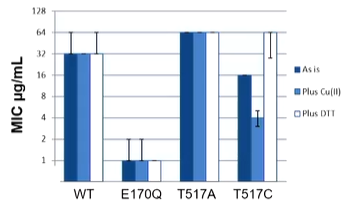
Two conformations with a large parting of the helices on the periplasmic side. The idea is to lock the molecule by preventing opening, you should see inactivity of the proteon. The ATP free state is 14 angstroms apart with Thr517 being the important residue. These are less than 3 angstroms when they are close. If these are swapped for cysteines, a disulfide bond can be formed which will lock the molecule and then the resistance to erythromycin can be measured:
Mechanotransmission describes how MacN like ABC transporters couple cytoplasmic ATP binding and hydrolysis with periplasmic conformational changes. This is described to work in a bellow like motion. When the nucleotide free MacB has an antibiotic enter it, ATP binding essentially compresses the protein to push the antibiotic out. Equilibration brings the protein back to its resting ATP bound state. ATP hydrolysis then resets the system