Chapter 21: NUCLEAR CHEMISTRY
Radioactivity and Nuclear Equations
- Nucleons — Protons and neutrons; the two types of subatomic particles that reside in the nucleus.
- All atoms of a given element have the same number of protons; this number is the element’s atomic number.
- Mass number — The total number of nucleons in the nucleus.
- Isotopes — Atoms with the same atomic number but different mass numbers.
- Radionuclides — Nuclides that are radioactive.
- Radioisotopes — Atoms containing radionuclides.
Nuclear Equations
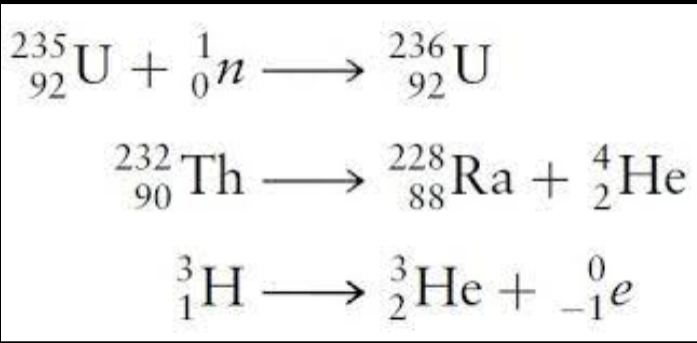
- Most nuclei in nature are stable and remain intact indefinitely. However, radionuclides are unstable and spontaneously emit particles and electromagnetic radiation.
- Emission of radiation is one of the ways in which an unstable nucleus is transformed into a more stable one that has less energy. The emitted radiation is the carrier of the excess energy.
- When a nucleus spontaneously decomposes in this way, it is said to be radioactive and to have decayed or to have undergone radioactive decay.
- Because an alpha particle is involved in this reaction, scientists also describe the process as alpha decay or alpha emission.
Example of Nuclear Equation:
Types of Radioactive Decay
- Alpha Radiation — Consists of a stream of helium-4 nuclei known as alpha particles.
- Beta Radiation — Consists of streams of beta particles, which are high-speed electrons emitted by an unstable nucleus.
- Gamma Radiation — Consists of high-energy photons (that is, electromagnetic radiation of very short wavelength).
Positron Emission and Electron Capture
[ ] Positron — Is a particle that has the same mass as an electron (thus, we use the letter e and superscript 0 for the mass) but the opposite charge (represented by the +1 subscript).
The isotope carbon-11 decays by positron emission: \n
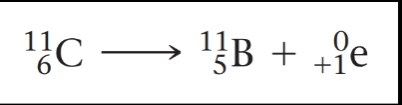
Positron emission causes the atomic number of the reactant in this equation to decrease from 6 to 5. Positron emission has the effect of converting a proton to a neutron, thereby decreasing the atomic number of the nucleus by 1 while not changing the mass number:
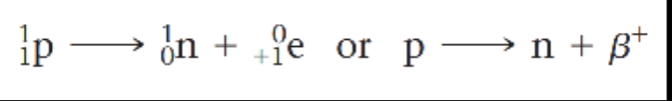
Electron capture is the capture by the nucleus of an electron from the electron cloud surrounding the nucleus, as in this rubidium-81 decay:

Because the electron is consumed rather than formed in the process, it is shown on the reactant side of the equation. Electron capture, like positron emission, has the effect of converting a proton to a neutron:
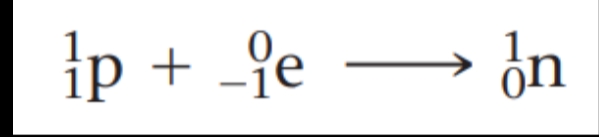
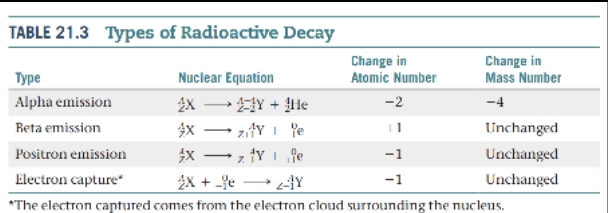
Patterns of Nuclear Stability
Neutron-to-Proton Ratio
- Strong nuclear force — A strong force of attraction at distances, existing between nucleons.
Three General Situations labeled as 1, 2 and 3 in the table.
Nuclei above the belt of stability (high neutron-to-proton ratios).
Nuclei below the belt of stability (low neutron-to-proton ratios).
Nuclei with atomic numbers ≥ 84.
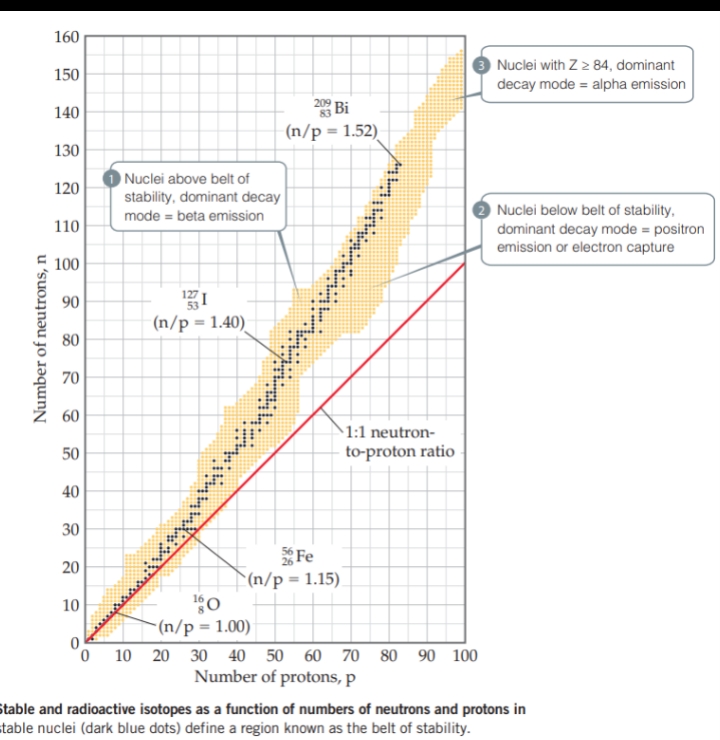
Radioactive Decay Chains
[ ] Radioactive decay chain — A series of nuclear reactions that begins with an unstable nucleus and terminates with a stable one; also known as nuclear disintegration series.
Two further observations can help us to predict stable nuclei:
- Nuclei with the magic numbers of 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons are generally more stable than nuclei that do not contain these numbers of nucleons.
- Nuclei with even numbers of protons, neutrons, or both are more likely to be stable than those with odd numbers of protons and/or neutrons. Approximately 60% of stable nuclei have an even number of both protons and neutrons, whereas less than 2% have odd numbers of both.
These observations can be understood in terms of the shell model of the nucleus, in which nucleons are described as residing in shells analogous to the shell structure for electrons in atoms.
\n Nuclear Transmutations
- [ ] Nuclear Transmutations — Occurs when a nucleus is struck by a neutron or by another nucleus which causes it to change its identity.
Accelerating Charged Particles
Alpha particles and other positively charged particles must move ver y fast to overcome the electrostatic repulsion between them and the target nucleus.
Many methods have been devised to accelerate charged particles, using strong magnetic and electrostatic fields.
Cyclotron — In this device, charged particles move in a spiral path within two D-shaped electrodes. Alternating charges on the electrodes accelerate the particles, while magnets above and below the device constrain the particles to a spiral path of increasing radius.
Synchrotron — In this device, the magnetic fields are synchronized so that the particle moves in a circular rather than a spiral path.
Reactions Involving Neutrons
- Most synthetic isotopes used in medicine and scientific research are made using neutrons as the bombarding particles.
Example:
Cobalt-60, which is used in cancer radiation therapy, is produced by neutron capture. Iron-58 is placed in a nuclear reactor and bombarded by neutrons to trigger the following sequence of reactions:
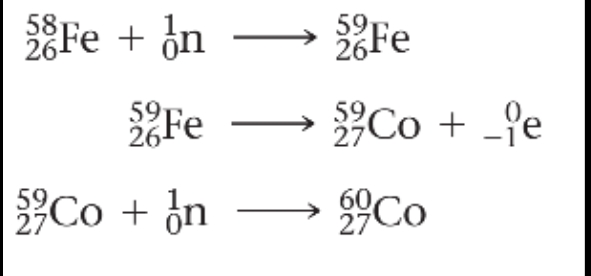
Transuranium Elements
Nuclear transmutations have been used to produce the elements with atomic number above 92, collectively known as the transuranium elements because they follow uranium in the periodic table.
Elements 93 (neptunium, Np) and 94 (plutonium, Pu) were produced in 1940 by bombarding uranium-238 with neutrons:
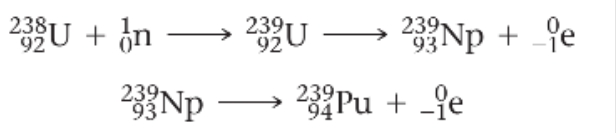
International Union for Pure and Applied Chemistry (IUPAC) — The international body that authorizes names of new elements after their experimental discovery and confirmation.
- According to the IUPAC, new elements can be named after a mythological concept, a mineral, a place or country, a property, or a scientist.
Rates of Radioactive Decay
Radioactive decay — a first-order kinetic process.
- A first-order process has a characteristic half-life, which is the time required for half of any given quantity of a substance to react.
Radiometric Dating
- [ ] Radiometric dating — The method of dating objects based on their isotopes and isotope abundances.
- [ ] Radiocarbon dating — The technique of dating when carbon-14 is used in radiometric dating.
Calculations Based on Half-Life
Activity — The rate at which a sample decays and it is often expressed as number of disintegrations per unit of time.
Becquerel (Bq)— The SI unit for expressing activity. It is defined as one nuclear disintegration per second.
Curie (Ci )— An older unit of activity, defined as 3.7 x 10^10 disintegrations per second, which is the rate of decay of 1 g of radium.
As a radioactive sample decays, the amount of radiation emanating from the sample decays as well.
A 4.0-mCi sample of cobalt-60 undergoes — and so has an activity of 1.5 x 10^8 Bq. \n

Detection of Radioactivity
Radiotracers
The use of radioisotopes is possible because all isotopes of an element have essentially identical chemical properties.
When a small quantity of a radioisotope is mixed with the naturally occurring stable isotopes of the same element, all the isotopes go through the same reactions together.
The element’s path is revealed by the radioactivity of the radioisotope.
Because the radioisotope can be used to trace the path of the element, it is called a radiotracer.
Energy Changes in Nuclear Reactions
Nuclear Binding Energies — The energy required to separate a nucleus into its individual nucleons.
Mass Defect — The mass difference between a nucleus and its constituent nucleons
[ ] Fission: Happens when heavy nuclei gain stability and therefore give off energy if they are fragmented into two midsized nuclei.
[ ] Fusion: Happens when the sharp increase in the graph for small mass numbers, even greater amounts of energy are released if very light nuclei are combined, or fused together, to give more massive nuclei.
\n Nuclear Power: Fission
- Nuclear fission is the process used to generate energy in nuclear power plants.
- Chain Reactions — Reactions that multiply.
- Critical Mass — The amount of fissionable material large enough to maintain a chain reaction with a constant rate of fission.
- When a critical mass of material is present, one neutron on average from each fission is subsequently effective in producing another fission and the fission continues at a constant, controllable rate.
- Supercritical Mass — A mass in excess of a critical mass.
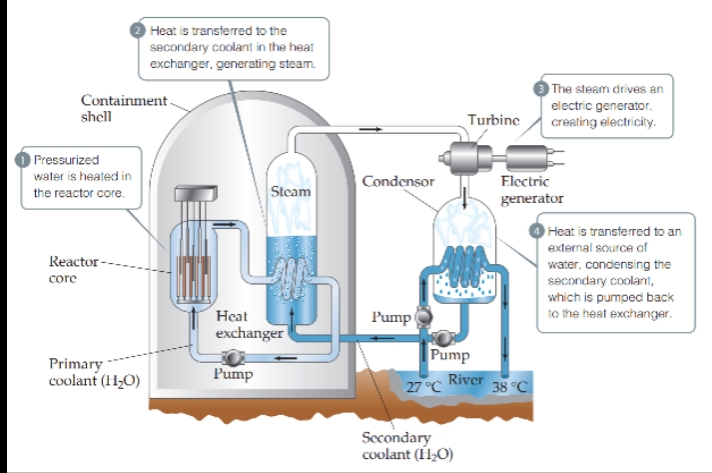
Nuclear Reactors
- Nuclear power plants use nuclear fission to generate energy.
- The core of a typical nuclear reactor consists of four principal components:
- Fuel Elements — Contain enriched uranium in the form of UO2 pellets encased in zirconium or stainless steel tubes.
- Control Rods — Composed of materials that absorb neutrons, such as boron-10 or an alloy of silver, indium, and cadmium. These rods regulate the flux of neutrons to keep the reaction chain self-sustaining and also prevent the reactor core from overheating.
- Moderator — It functions to slow down the neutrons (to speeds of a few kilometers per second) so that they can be captured more readily by the fissionable nuclei. It is typically either water or graphite.
- Primary Coolant — A substance that transports the heat generated by the nuclear chain reaction away from the reactor core.
Pressurized Water Reactor — The most common commercial reactor design wherein the water acts as both the moderator and the primary coolant.
\n Several Variations of Pressurized Water Reactor
- Boiling Water Reactor — Generates steam by boiling the primary coolant; thus, no secondary coolant is needed.
- Heavy Water Reactor — Uses Deuterium as moderator and primary coolant.
- Gas Cooled Reactor — Uses a gas, typically CO2, as primary coolant and graphite as the moderator.
Nuclear Waste
When the fuel elements are removed from the reactor, they are initially very radioactive. It was originally intended that they be stored for several months in pools at the reactor site to allow decay of shortlived radioactive nuclei. They were then to be transported in shielded containers to reprocessing plants where the unspent fuel would be separated from the fission products.
[ ] Fast Breeder Reactor — Offers one approach to getting more power out of existing uranium sources and potentially reducing radioactive waste.
This type of reactor is so named because it creates (“breeds”) more fissionable material than it consumes.
The reactor operates without a moderator, which means the neutrons used are not slowed down.
Nuclear Power: Fusion
[ ] Fusion is appealing as an energy source because of the availability of light isotopes on Earth and because fusion products are generally not radioactive.
The problem is that extremely high temperatures and pressures are needed to overcome the electrostatic repulsion between nuclei in order to fuse them.
Thermonuclear Reactions — also known as Fusion Reactions.
- The lowest temperature required for any fusion is about 40,000,000 K, the temperature needed to fuse deuterium and tritium.
- Such high temperatures have been achieved by using an atomic bomb to initiate fusion. This is the operating principle behind a thermonuclear, or hydrogen, bomb.
- Numerous problems must be overcome before fusion becomes a practical energy source. In addition to the high temperatures necessary to initiate the reaction, there is the problem of confining the reaction. No known structural material is able to withstand the enormous temperatures necessary for fusion. \n
Radiation in the Environment and Living Systems
[ ] Ionizing Radiations — Radiation that causes ionization which is far more harmful to biological systems than radiation that doesn’t cause ionization.
[ ] Nonionizing Radiations — Much lower energy than ionizing radiations, such as radiofrequency electromagnetic radiation or slow moving neutrons.
[ ] Free Radical — A substance with one or more unpaired electrons, as seen in the Lewis structure shown in the margin.
In light of the biological effects of radiation, it is important to determine whether any levels of exposure are safe.
Any amount of radiation is assumed to cause some finite risk of injury, and the effects of high dosage rates are extrapolated to those of lower ones.
Radiation Doses
[ ] Gray (Gy) — The SI unit of absorbed dose, corresponds to the absorption of 1 J of energy per kilogram of tissue.
[ ] Rad (Radiation Absorbed Dose) — Corresponds to the absorption of 1 X 10^–2 J of energy per kilogram of tissue. It is most often used in medicine.
[ ] RBE (Relative Biological Effectiveness): The radiation dose is multiplies by a factor that measures the relative damages caused by the radiation. It is approximately 1 for gamma and beta radiation, and 10 for alpha radiation.The exact value of the RBE varies with dose rate, total dose, and type of tissue affected. The product of the radiation dose in rads and the RBE of the radiation give the effective dosage in REM (roentgen equivalent for man):
Number of rem = (number of rad) (RBE)
Sievert (Sv) — SI unit for effective dose.
\n