A.2.2.1 (2) Chemical composition of macronutrients
Carbohydrates (Saccharides)
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen in a ratio of 1:2:1, typically represented as (CH₂O)n.
This structure allows carbohydrates to serve as a primary energy source for living organisms, with simple sugars like glucose and complex carbohydrates like starches and fibres playing crucial roles in metabolism.
They are classified into monosaccharides, disaccharides, and polysaccharides based on their molecular structure and complexity. Their general formula is CH2O, where n is the number of carbon atoms in the molecule.
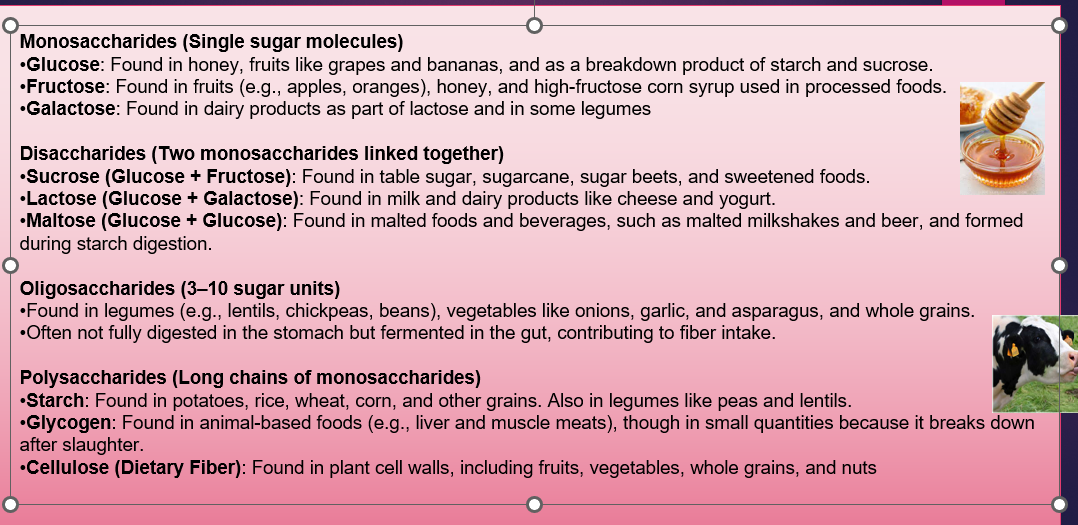
Monosaccharides are the simplest form made of one molecule and are easily absorbed by the human body (e.g. glucose, fructose and galactose)
Food sources for monosaccharides: Honey, Dried fruits
Disaccharides are two monosaccharides that form disaccharides with the loss of a water molecule (e.g. fructose is combined with glucose to create sucrose, while lactose consists of glucose and galactose.)
Food sources for monosaccharides: Sugar cane, sugar beet, dairy
Oligosaccharides are carbohydrates with three to nine molecules for example, meltadoxin
Food sources for monosaccharides: Vegetables, beans, legumes
Polysaccharides are molecule chains longer than 10 molecules for example, starch and glycogen
Food sources for monosaccharides: Bread, potatoes, cereal, corn, grains, rice
Lipids (Fatty acids and glycerol)
One triglyceride (fats/lipids) comprises one glycerol molecule and three fatty acids. They are a chain of:
Carbon atoms
Hydrogen attached
Methyl group (CH3)
Carboxyl Group (COOH)
Proteins
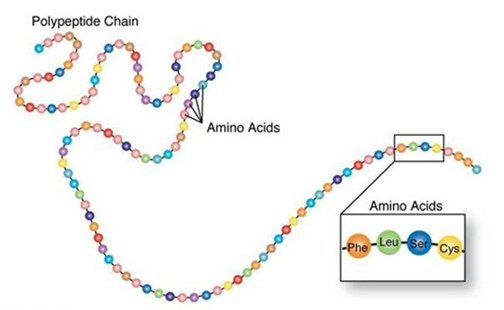
They are formed by amino acids
Made of carbon, nitrogen, oxygen and hydrogen
Each protein has an amino acid composition
20 amino acids have been identified as being required for the synthesis of proteins. 9 are essential (need to be provided in the diet)
Why 20 amino acids?
Your body needs 20 specific amino acids to create all the proteins it needs:
9 are essential, meaning that they must be retrieved from our diet as the body can’t make them
The other eleven are non-essential because your body can produce them on its own
Glycogen
Glycogen is a storage form of glucose. Glucose is converted into glycogen when glucose levels are too high. Glycogen is formed by two glucose molecules combining to make a disaccharide via the condensation reaction. Repeated processing results in a polysaccharide.
Storage = Muscles and liver
Condensation reaction
The condensation reaction involves the removal of water. When two monosaccharides combine, water is removed, giving us a glycosidic bond, creating a disaccharide. This can happen more than once to create oligo- and polysaccharides
Key Words
Metabolism: All the biochemical reactions that occur within an organism, including anabolic and catabolic reactions
Anabolism: Energy-requiring reactions whereby small molecules are built
Catabolism: Chemical reactions that break down complex organic compounds into simpler ones
Aerobic and Anaerobic Catabolism
Aerobic Catabolism/Aerobic energy system: breakdown of molecules in the presence of oxygen to release energy
Key process: Cellular respiration
Products: CO2 and water and a lot of ATP
Efficiency: High ATP yield (36-38 ATP per glucose molecule)
Location: In the mitochondria
Example: Long-distance running or moderate-intensity exercise
Anaerobic Catabolism: breakdown of molecules in the absence of oxygen
Key process: Glycolysis is formed by fermentation
Products: Lactic acid (animals) or ethanol and carbon dioxide (in yeast), and little ATP
Efficiency: Low ATP yield
Location: Cytoplasm
Example: Sprinting or high-intensity, short-duration exercises
