Matter & Measurement Notes
What is Chemistry/Matter?
What is Chemistry?
Chemistry is the study of composition, structure, and properties of matter, the processes that matter undergoes, and the energy changes that accompany these changes.
What is Matter?
Matter has mass and takes up space
Everything around you is composed of matter
What is not matter: (energy, light, heat, sound, etc.)
There are 3 states of matter we focus on in this class
Gas
Liquid
Solid
States of Matter
Each addition of energy creates a change in the state of matter
More energy = more movement = change in state of matter
States of Matter from least to most energy
Bose-Einstein Condensate
Occurs when objects reach absolute zero temperature
Atoms become waves, then become one whole entity
Solid
Liquid
Gas
Plasma
Lightning, Aurora, Gas in neon signs
Basics of Matter & Vocabulary
Volume: Amount of 3D space an object occupies
Mass: Measures the amount of matter
Matter: Has mass and takes up space
Weight: Pull of gravity on matter
Atom: Smallest unit of an element
Element: Pure substance made of one type of atom
Compound: Pure substance made of 2 or more elements that are chemically bonded
Example: NaCI, H2O, CaCO3
Molecule: Pure substance made of 1 element, chemically bonded
Example: H2, O2, S8
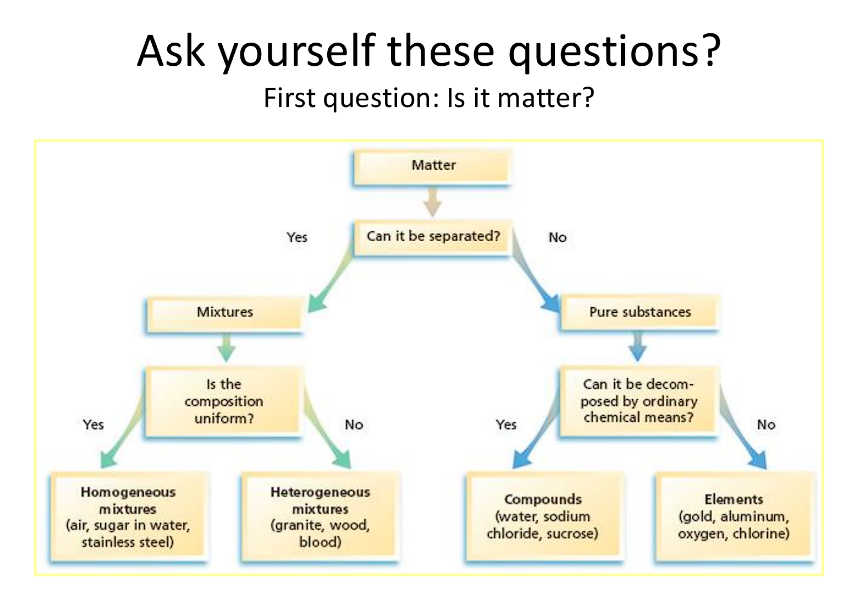
Pure Substance: Consists of 1 type of matter
Element, Compound, Molecules
Mixture: 2 or more types of matter
Can be a homogeneous or heterogeneous mixture
Homogeneous Mixture:
Same Throughout
Looks like one mixture
Uniform with no visual difference throughout
Examples: Vodka, Steel, Air, Rain
Heterogeneous Mixture:
Different appearance throughout
Can see different parts of mixture
Examples: Cereal in milk, ice in soda, soil, sand
Mixtures
Methods to separate mixtures:
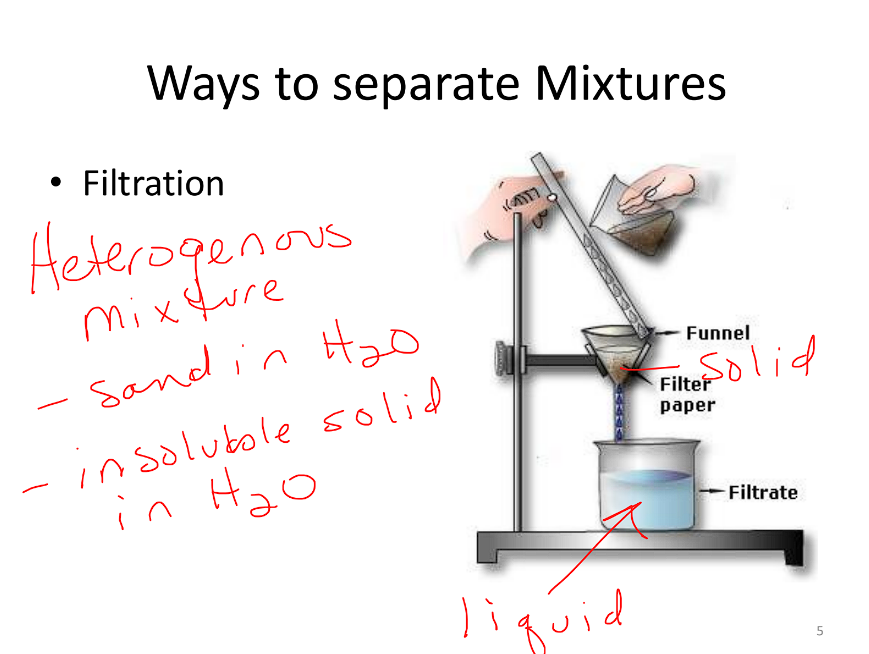
Filtration
Separates heterogeneous
Examples:
Sand in water
Any insoluble solid in water
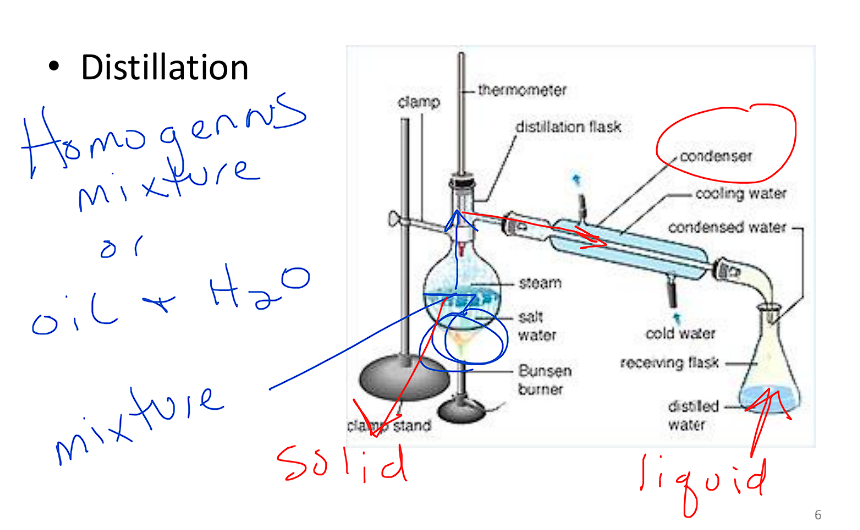
Distillation
Can separate homogeneous
Also separates oil and water
Heats up the mixture
Liquid becomes gas
Gas travels through tube into container
Gas condenses into water in separate container
Evaporation can be done instead but doesn’t preserve the water
Evaporation
Separates Homogeneous
Same as distillation but doesn’t save the liquid
Only solid remains
Practice:
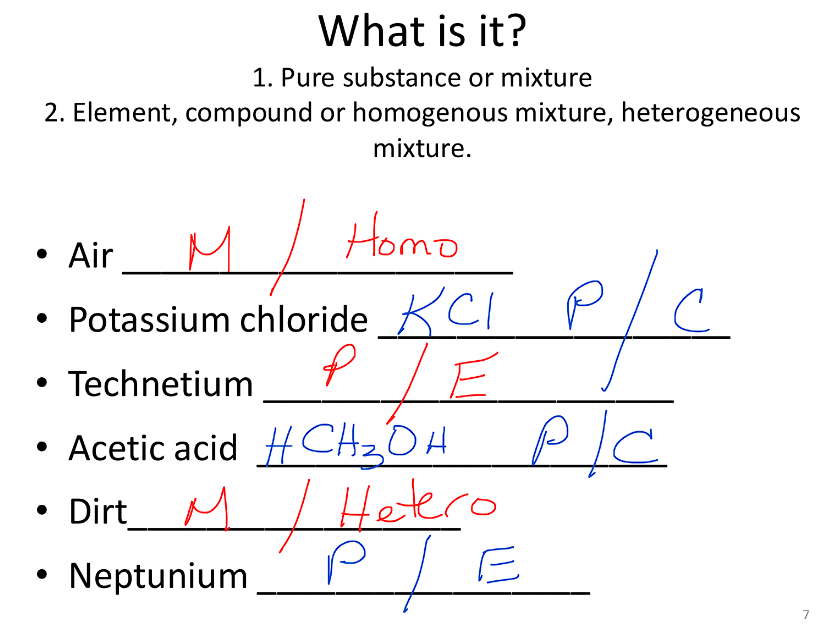
How to identify matter:
Physical & Chemical Properties of Matter
Physical Changes: Matter does not change
May look different
Same matter
Salt in water, still salt
Coloring on paper, still paper
Chemical Changes: Changes 1 substance into something else
Couldn’t change substances that have undergone a chemical change back to their original form through distillation or filtering
Different substance is formed
How to identify the type of change:
Signs of a chemical change
Evolution of bubbles
Precipitate formed - insoluble solid in water
Heat or light released
Color change
Usually use more than 1 sign to identify if a change occurred

Examples of chemical properties of matter (Extensive Properties)
Ability to react with oxygen
Ability to react with acids
Reacts with water
Does not react with oxygen
Does not react with acids
Does not react with water
Examples of physical properties of matter (Intensive Properties)
Boiling point
Melting point
Description
Color
Hardness
Malleability - ability to form into sheets
Ductility - Ability to form into wires
Density
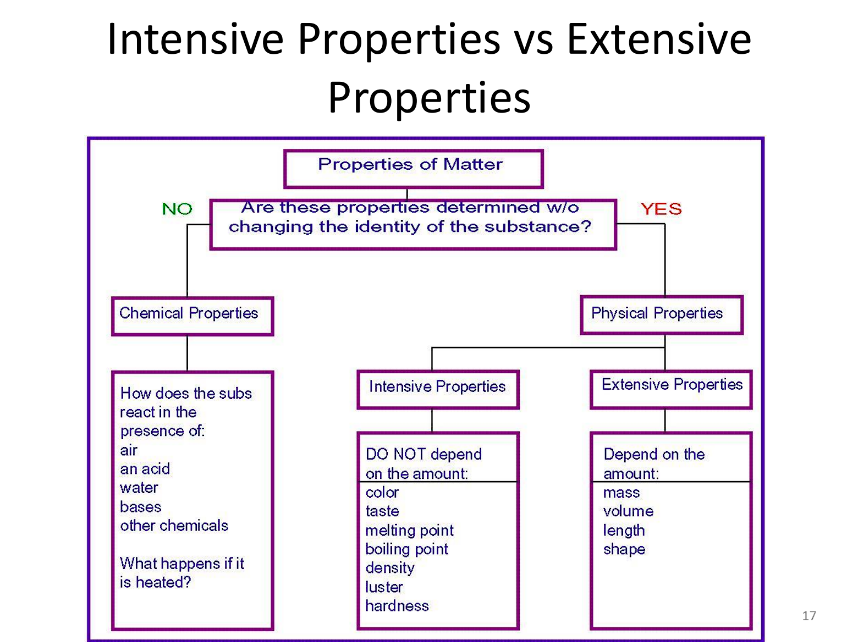
Intensive vs Extensive Properties
Intensive Property:
Does not depend on the amount of substance present.
Examples: Density, boiling point, color, temperature.
Determination: Measure the property in a sample; if it remains constant regardless of sample size, it is intensive.
Extensive Property:
Depends on the amount of substance present.
Examples: Mass, volume, total energy, length, shape
Determination: Measure the property in a sample; if it changes with the size of the sample, it is extensive.
Sig Figs Notes
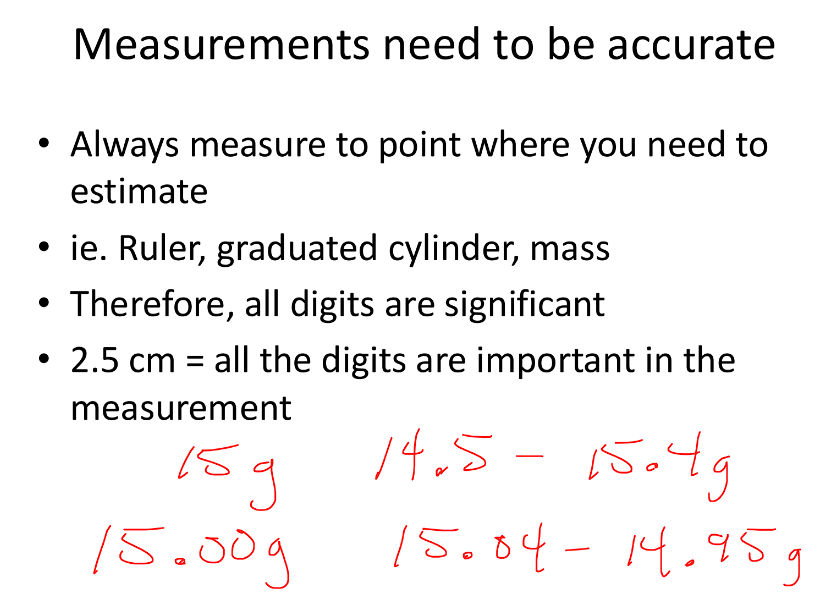
Importance of Measurement Accuracy
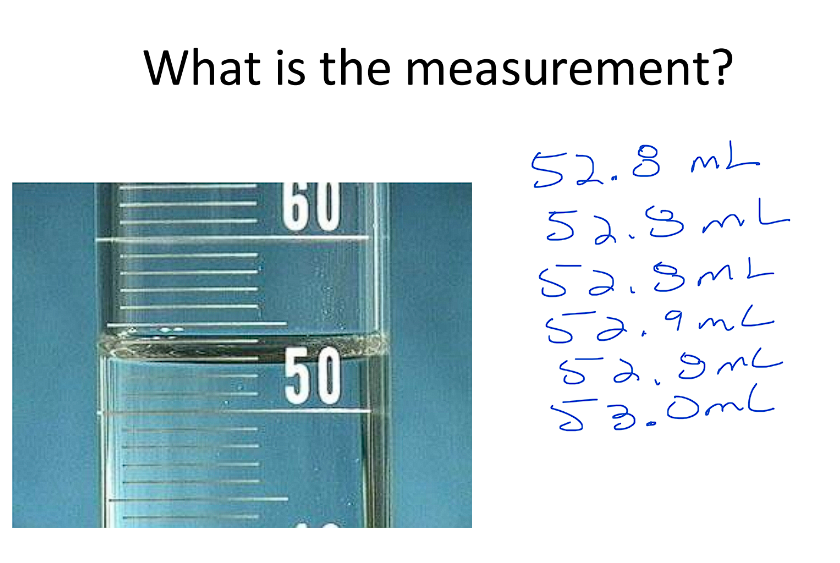
Example:

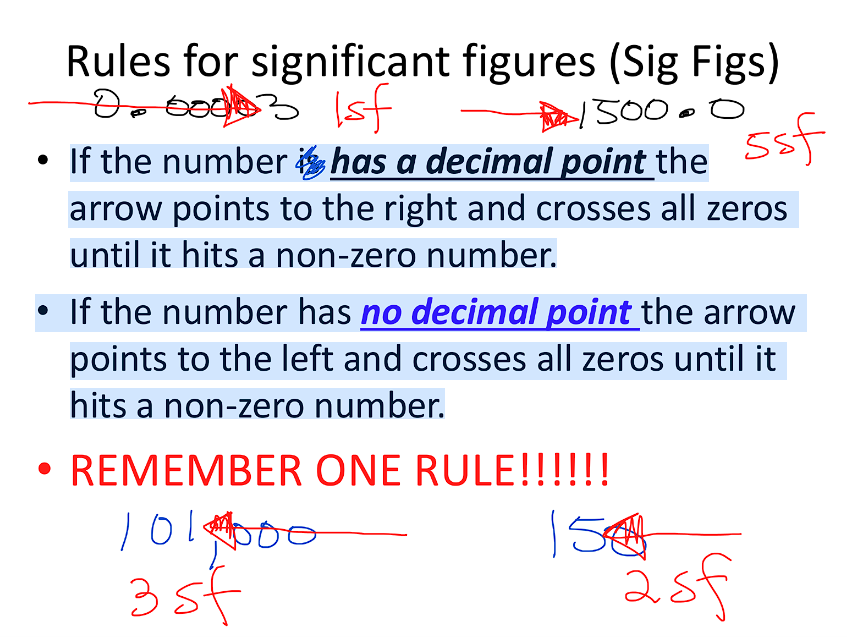
Significant Figures Rules
All non-zero numbers significant
ZERO’S
Leading: never count (ie. 0.0000003 = 1 sig fig)
Captive: always count (ie. 1.0000045 = 8 sig fig)
Trailing: only count if number has a decimal point
100 = 1 sig fig
100. = 3 sig fig
150.0 = 4 sig fig
Exact numbers – unlimited significant figures
Examples 1 atm = 14.7 psi
Simpler Rules
If the number is has a decimal point the arrow points to the right and crosses all zeros until it hits a non-zero number.
If the number has no decimal point the arrow points to the left and crosses all zeros until it hits a non-zero number.
Example:

Rounding, Addition, Subtraction, Multiplication and Division Rules


Scientific Notation:
Used to write very small or very large numbers
Writes numbers as a decimal multiplied by 10 to the power of something
Examples
253,000,000 = 2.53×10^8
0.000047 = 4.7×10^-5
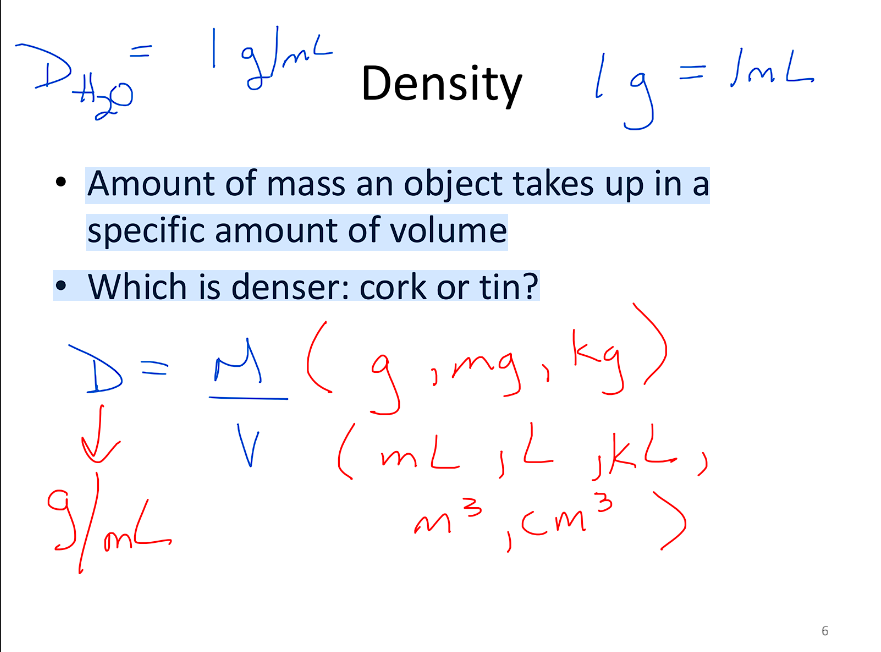
Density
Amount of mass an object takes up in a
specific amount of volume
Formula: Density = Mass/Volume (D=M/V)
Which is denser: cork or tin?

Factor Label Notes
Qualitative Data: Interpretation based, descriptive, or relating to language
Quantitative Data: Numbers based, countable, or measurable
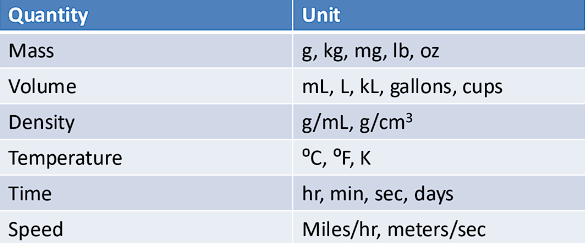
Quantities vs. Units
SI System
SI system created to have a standard set of units used for measurements
Mass = kg
Length = m
Time = s
Temperature = K
Metric System Prefixes
Prefix | Abbreviation | Meaning | Power of 10 |
giga- | G | 1,000,000,000 | 109 |
mega- | M | 1,000,000 | 106 |
kilo- | k | 1,000 | 103 |
hecto- | h | 100 | 102 |
deka- | da | 10 | 101 |
deci- | d | 0.1 | 10-1 |
centi- | c | 0.01 | 10-2 |
milli- | m | 0.001 | 10-3 |
micro- | μ | 0.000001 | 10-6 |
nano- | n | 0.000000001 | 10-9 |
pico- | p | 0.000000000001 | 10-12 |